Document 5751306
advertisement

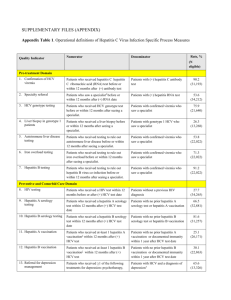
Prabhdeep Sidhu Candidate PharmD 2015 Western University of Health Sciences Mar 28, 2014 Objectives Discuss epidemiology of Hepatitis C Explain testing and diagnosis Describe current treatments Explain new medication Sovaldi Identify place in treatment Compare Sovaldi with other treatment plans Patient Case AJ is 45 year old male African American patient who got diagnosed with Hepatitis C genotype 2. AJ has a past medical history of DM and is taking metformin and glipizide. Pt also has a history of IVDU and stopped using drugs 5 years ago. Pt is experiencing fatigue and low in energy from past few months. Resident physician wants to start AJ on Sovaldi and asking for pharmacist’s receommendation. Hepatitis C Symptoms Acute Mostly asymptomatic Chronic Persistent fatigue Nausea Poor appetite Transmission Caused by infection with Hepatitis C virus Spread through exposure to infected blood Sharing needles to inject drugs Blood transfusions (before 1992) Hepatitis C Approximately 3.2 million people infected with HCV Risk groups Injection drug users Recipient of blood products prior to 1992 Chronic haemodialysis patients Persons with HIV infection Children born to HCV infected mothers Testing Recommendations Persons born from 1945 through 1965 Persons injecting illegal drugs Recipients of blood transfusions prior to 1992 Long term haemodialysis patients Persons with HIV infection Diagnosis Screening tests for antibody to HCV RNA polymerase chain reaction Quantitative test to detect amount of virus Current Treatments For genotype 1 (duration varies on treatment response) Peg interferon and Ribavirin Plus Boceprevir or Telaprevir For genotype 2 or 3 ( 24 weeks) Peg interferon Plus Ribavirin Sovaldi (Sofosbuvir) Nucleotide analogue inhibitor of hepatitis C virus NS5B polymerase enzyme 400 mg tablet Once daily Approved Dec 2013 Mechanism of Action HCV NS5B RNA dependent RNA polymerase Essential for viral replication Sovaldi is a prodrug which undergoes intracellular metabolism to active form uridine analog triphospahte Gets incorporated into viral RNA and acts as a chain terminator Pharmacokinetics Parameters Sofosbuvir Absorption Tmax – 0.5 to 2 hrs Distribution 61% to 65% protein bound Metabolism Extensively metabolised Excretion Primarily renal (80%) Elimination Half-life Parent compound 0.4 hours Active metabolites 27 hours Adverse Effects Common Rash (18%) Diarrhea (12%) Nausea (34%) Anemia (6%) Serious Pancytopenia (<1%) Increased bilirubin levels (3%) Depression (<1%) Monitoring Detect viral load Improvement in signs and symptoms of Hepatitis C In female patients and female partners of male patients, perform pregnancy test before initiating treatment and monthly thereafter, and for 6 months after discontinuing treatment CI and DDI CI Pregnancy – Category B DDI Carbamazepine Fosphenytoin Oxcarbazepine Phenytoin Rifampin St. John’s wort Place in treatment Genotype 1 Sovaldi + Peg interferon + Ribavirin for 12 weeks Genotype 2 Sovaldi + Ribavirin for 12 weeks Genotype 3 Sovaldi + Ribavirin for 24 weeks Treatment comparison NEUTRINO trial – single group open label trial Outcome Sovaldi + Ribavirin + Peg- inerferon for 12 weeks (N= 327) SVR in Genotype 1 HCV RNA < 25IU/ml 89% Anemia 23% Leukopenia 5% Thrombocytopenia 1% Treatment comparison FISSION trial – randomized open label active- control groups Outcome Sovaldi + Ribavirin for 12 weeks (N = 256) Peg- interferon + Ribavirin for 24 weeks (N = 243) SVR in Genotype 2 HCV RNA < 25IU/ml 67% 67% Anemia 9% 14% Leukopenia 0% 4% Thrombocytopenia 0% 7% Treatment comparison Cost Peg- interferon + Ribavirin for 24 weeks = 23,002 Sovaldi + Ribavirin for 12 weeks = 101, 195 Pros &Cons Pros Oral Administration Once daily dosing Shorter treatment duration Cons – Increase in cost of treatment Long term data not available Recommendation for AJ Peg-interferon 180 mcg once weekly for 24 weeks Ribavirin 400 mg twice daily for 24 weeks References Hepatitis C information for health professionals. Retrieved on Mar 24, 2014 from http://www.cdc.gov/hepatitis/HCV/index.htm Sovaldi Lexi-comp http://online.lexi.com.proxy.westernu.edu/lco/action/doc/retrieve/docid/patch_f/4884323 Accessed on Mar 27, 2014. Lawitz E. et al. Sofosbuvir for previously untreated chronic hepatitis C infection. The New England Journal of Medicine 2013; 368:1878-1887 Thank You Questions