Single-breath
advertisement

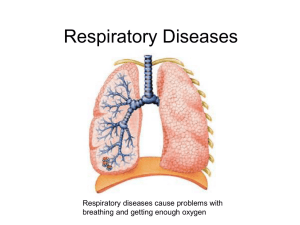
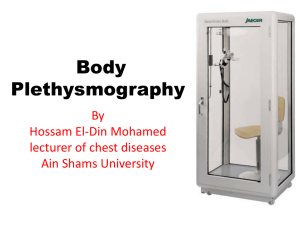
Measuring Lung Volumes and AHR James Zangrilli MD Topics • Measuring lung volumes by tracer gas dilution – Single breath – Multiple breath – Nitrogen washout • Plethysmography • Airway hyperresponsiveness – Physiologic basis – Measurement SLE with DOE and orthopnea FVC 1.22 L (pred 3.79) Reasons to measure Lung Volumes? • To confirm/support your clinical diagnosis of restrictive airway disease – Hx, physical, radiograph, low FVC with FEV1/FVC (> ~ 85%) • Utility in obstruction? – Gas trapping: ‘nice to know’ • To ascribe some level of severity to the restriction after you decide the test is abnormal – % predicted • Monitor disease progression or response to treatment When is lung function value is abnormal? • Based on percent predicted values (i.e. w.i. 80-120%) – common but deemed less rigorous • A restrictive ventilatory defect is characterised by a reduction TLC below the 5th percentile of the predicted value, and a normal FEV1/VC. ATS-ERS Interpretive Strategies, Eur Respir J 2005; 26: 948–968 http://www.spirxpert.com/expressing3.htm Subdivisions of lung volume Calculating lung volume by gas dilution • Wash-in: diluting a tracer gas in a lung compartment – Breathing helium mix via a closed circuit spirometer • Single breath helium dilution includes a vital capacity breath hold – Included as part of the DLCO • Multiple breath helium dilution: rebreathing of the helium mix at FRC until the lung and spirometer have equilibrated – Determination of lung volume is based on • knowing the initial volume of gas in the spirometer – Or VI for for single breath technique • the amount of helium dilution that has occurred during the test. • Wash out: washing gas out of the lung compartment – utilize endogenous nitrogen as “tracer” Tracer gas • Properties of a good tracer – Should be relatively insoluble and biologically inert • Does not leave the alveoli – Not normally present in the alveolar gas • E.g. He, CH4 Single breath helium dilution and VA • The amount of tracer inspired = the final amount in lung • Vi·Fi,He = VD·Fi,He+ VA·FA,He F • VA = (Vi - VD) · i,He FA,He Estimated (2.2ml/kg IBW) Volume of gas inspired VI – Rapid inhalation from RV to TLC Multi-breath helium dilution technique and TLC • Person breathes in and out of a spirometer filled with a mixture of helium and oxygen until the exhaled and inhaled He concentrations reach equilibrium • The test is stopped at the end of a normal tidal expiration (i.e. at the FRC) CLOSED CIRCUIT Multi-breath helium dilution technique and TLC • The total volume of helium initially = total volume at the end of the test – Vsp • FHei = FHef (Vsp + Vfrc) Nitrogen washout technique for lung volume determination • Breath 100% O2 in and out through a one-way valve (open circuit) – collect all of the exhaled breath until the exhaled nitrogen registers 0 (i.e. FRC washed out) • The volume of N2 in the lung initially = volume of N2 washed out • 0.8 FRC = FN2 final·V2 O2 – FRC = 1.25·FN2·V2 final N2 Ni V2 VA vs TLC • Total Lung Capacity (TLC) is routinely measured with the multiple-breath closed-circuit helium dilution technique. • Single-breath helium dilution provides an estimate of TLC, commonly referred to as alveolar volume (VA). – VA is automatically included with measurement of DLCO – relatively rapid and simple • VA can underestimate the multiple-breath TLC in patients with obstructive lung disease – Helium may not mix thoroughly in 10 seconds – All gas dilution techniques can underestimate volume in slowly emptying/noncommunicating lung VA underestimates TLC as degree of obstruction worsens …but its pretty close in normal and restricted subjects where VA + VDest ≈ TLC Chest Punjabi et al. 1998, 114 (3): 907 Thoracic Gas Volume via plethysmography • Volume of gas contained in the thorax whether communicating or not • Principle: Boyles Law P1V1=P2V2 – Closed container Mouth pressure FLOW Panting against a closed shutter • compresses and decompressed the lung • reciprocal change in the body box pressure corresponds to → ΔV Box Pressure Transducer P1·FRC = P2 (FRC+ ΔV) FRC = P2 ΔV P1-P2 Calibration syringe Body box bonus: gives airway resistance • Obstruction can be assessed by – Flow during conditions of maximal effort (spirometry) – Airway resistance under low flow conditions • Panting with shutter open gives airflow at the mouth (Vm) • Occlude the airway: record pressure at the mouth ≈ alveolar pressure Palv Raw = Vm Airway hyperresponsiveness Definition of AHR • A condition in which the airway reacts too readily and too much to nonspecific bronchoconstricting stimuli – Reflective of the variable airway obstruction seen in asthma • exercise, cold, irritant, etc (a specific stimulant would be allergen) – Mimic in the lab with bronchial challenge • AHR is closely associated with - but not identical to - the diagnosis of asthma – Common in COPD and can be seen in other diseases Reasons to measure AHR • Adjunct to initial asthma assessment – Help to determine if asthma is present or not – Special situations: Cough, occupational • Exploratory endpoint for research – Novel drug trials (particularly early phase clinical) – Common in preclinical asthma studies • Emerging: a complement to asthma control assessment in treated patents – Potential to guide therapy ARH and airway structure Circular bands of smooth muscle surround the airway epithelium as far peripherally as the respiratory bronchioles Physiologic coupling of the parenchyma to the AWSM • Healthy lungs – In vivo evidence that airway diameter varies in proportion to lung volume – Suggests that airway smooth muscle, airway wall, and lung parenchyma are mechanically coupled Remodeling in asthma: changes in lung volume have diminished effect on airway diameter or ASM contractility • Structural remodeling – SM hypertrophy – Subendothelial thickening – Matrix alteration – Epithelial abnormlality • Inflammation Persistent and variable factors affect AHR Remodeling Epithelial dysfunction Inflammatory mediators • Mast cell • Inflammatory cells • Neuroendocrine cells Busse W W Chest 2010;138:4S-10S How structure is believed to affect AHR Remodeling: Inflammation injury repair – Subepithelial thickening – Proteases: decreased stiffness of the elastic elements – Smooth muscle hypertrophy Loss of effective tethering between muscle cells and dynamic physical environment Adaptation of the shortened muscle to its new length in chronic disease – Increased ability of the shortened muscle to generate force Measuring airway hyperresponsivness by provocation • • Persistent component primarily reflects smooth muscle function: sensitive to agonists that act directly on SM receptors • Relatively low dose needed • Airway caliber important Variable component critically depends on presence of inflammation with attendant mediator release – Clinical correlates: drying of the airways during exercise, cold air etc. Assessing airway hyperresponsiveness • Four hypothetical dose response curves for a smooth muscle agonist • Degree of hyperresponsiveness characterized by – Too readily: Ease of the response (sensitivity): leftward shift in PC20 – Too much: threshold typically set at ≥ 20% fall in FEV1 When is disease present? Sensitivity and specificity of histamine PC20: 500 randomly selected college students, 17 with current asthma Sx • For current symptomatic asthma as the diagnosis and PC20≤ 8 mg/ml – Sensitivity 100% – Specificity 93% – Negative predictive value 100% – PPV only 39% Strength is high sensitivity: i.e. PC20 ≥ 8 mg/ml likely to indicate that current asthma not present PC20 ≤ 8 Cockroft DW, JACI1992 ;89:23-30. More general population: cut-offs for methacholine* bronchoprovocation • Sensitive test with high negative predictive value for asthma – False negative ~16% - consider repeat if suspicion high • Relatively non-specificity unknown Am J Respir Crit Care Med Vol 161. pp 309-329, 2000 *Histamine ≈ methacholine Direct (structure) and indirect (inflammation) comparisons • • Direct challenge: (arbitrary) cut points define a highly sensitive, less specific test – Useful to r/o asthma Indirect challenge: studies support high specificity for asthma but low sensitivity relative to methacholine – Useful to confirm asthma Cockroft DW, Chest 2010;138;18S-24S Indirect bronchoprovocation using dry powder mannitol inhalation (initial trial) • Mannitol induced bronchoconstriction correlated well with hypertonic saline (n=43) and MeCh (n=25) challenge in asthma patients • Well tolerated • Spontaneous recovery of FEV1 wi 60 minute (10 with SABA) Major advantages: • greater specificity for detecting disease driven by inflammation • ease of use Anderson SD, AM J RESPIR CRIT CARE MED 1997;156:758–765 Mannitol believed to increased airway surface osmolarity leading to the release of inflammatory mediators from inflammatory cells and sensory nerves • Mannitol induced bronchoconstriction was inhibited by nedocromil – Similar to other drying (e.g. exercise) and osmotic challenge (e.g hypertonic saline) Brannan JD, Am J Respir Crit Care Med 2000, 161: 2096–2099, Mannitol AHR as a potential marker of asthma control Tracks with ICS usage Distinguished ICS step-down failures Correlates well with EIB Leuppi JD Am J Respir Crit Care Med Vol 163. pp 406–412, 2001 Brannan JD Am J Respir Crit Care Med 1998;158:1120–1126 Brannan JD Respirology (2002) 7, 37–44 Aridol Bronchial Challenge Test • Indicated for the assessment of bronchial hyperresponsiveness in patients 6 years of age or older who do not have clinically apparent asthma • A positive response is achieved when the patient experiences a 15% reduction in FEV1 from (0 mg) baseline (or a 10% incremental reduction in FEV1 between consecutive doses). The test result is expressed as a PD15.