the Powerpoint Presentation
advertisement

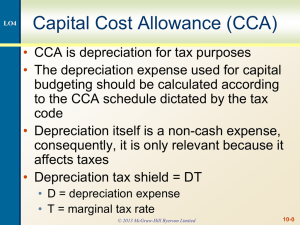
Update on Cholangiocarcinoma: What we have learned from the International Hepatobiliary Neoplasia Biorepository Roon Chaiteerakij, MD Mayo Clinic, Rochester, MN Chaiteerakij.roongruedee@mayo.edu Outline • International Hepatobiliary Neoplasia Biorepository (IHNB) • Studies on cholangiocarcinoma • How we use liver tissues collected from the IHNB to conduct research The IHNB Collects Data and Samples of Patients with Liver, Bile duct and Gallbladder Cancer and Controls Questionnaire Clinical data Tumor & Benign tissue Blood DNA plasma & serum Urine, Stool & Bile ©2013 MFMER | 3299636-3 Our Goal is to Improve Prevention, Diagnosis and Treatment of Liver, Bile Duct and Gallbladder Cancers Tumor Biology Clinical outcome Epidemiology study Hepatobiliary cancer Early diagnosis Personalized oncology Novel therapeutics ©2013 MFMER | 3299636-4 Epidemiology: Understanding the Risk Factors for Hepatobiliary Cancers Tumor Biology Clinical outcome Epidemiology study Cholangio carcinoma Early diagnosis Personalized oncology Novel therapeutics ©2013 MFMER | 3299636-5 Epidemiology Studies use Clinical Data, Risk Factor Questionnaires, and Blood Samples Questionnaire Clinical data Tumor & Benign tissue Blood DNA plasma & serum Urine, Stool & Bile ©2013 MFMER | 3299636-6 Current Epidemiology Studies on Cholangiocarcinoma (CCA) • Classification of CCA • Incidence of CCA • Clinical risk factors for CCA • Genetic risk factors for CCA Classification of CCA CCA is not a single disease but a group of three separate diseases The three have similarities, but also distinct differences Classification of Cholangiocarcinoma (CCA) 103 iCCA (intrahepatic) 71 pCCA (perihilar) Gallbladder Cystic duct Pancreas Image Courtesy of Dr. Gregory Gores 22 dCCA (distal) The Incidence Rate of Intrahepatic Cholangiocarcinoma in Olmsted County, MN, US has Increased 7-fold Incidence rates (Per 100,000 2.5 person-year) P trend = 0.02 2.1 2.0 1.5 1.0 0.5 0.8 0.3 0.0 1976-1990 Yang JD, et al. Am J Gastro 2012 1991-2000 Year 2001-2008 Classification of Cholangiocarcinoma (CCA) 103 iCCA (intrahepatic) 71 pCCA (perihilar) Gallbladder Cystic duct Pancreas Image Courtesy of Dr. Gregory Gores 22 dCCA (distal) The Incidence Rate of Distal CCA has Decreased by 35% Incidence rates (Per 100,000 2.5 person-year) 2.2 1.9 2.0 1.5 2.1 iCCA 1.5 pCCA 1.3 1.4 dCCA 1.0 0.5 0.8 0.3 0.6 0.0 1976-1990 Yang JD, et al. Am J Gastro 2012 1991-2000 Year 2001-2008 Demographics of 1267 CCA Patients Distribution of location (%) Proportion of Males 50% iCCA (Year) iCCA 60% 63% pCCA dCCA Mean age 61 62 iCCA pCCA 67 pCCA dCCA Data from IHNB dCCA Factors associated with iCCA development Risk (fold) 82 8 4 3 1.5 PSC Cirrhosis Diabetes Hepatitis C Smoking Primary Sclerosing Cholangitis Chaiteerakij, et al. Hepatology. 2013 Factors associated with iCCA development Risk (fold) 82 8 5 4 3 1.5 PSC Cirrhosis Diabetes Diabetes No Metformin use 2 Diabetes Metformin use Metformin use was associated with 60% reduction in risk for iCCA Chaiteerakij, et al. Hepatology. 2013 Study of Effect of Metformin Treatment on Cholangiocarcinoma in Mice Control Metformin Manuscript, in preparation Epidemiologic study • Current classification of CCA • Clinical risk factors for CCA • Genetic risk factors for CCA • Planned GWAS for CCA • Future directions Is genetic variation associated with risk of CCA development? A * G * Single nucleotide polymorphism (SNP) Is genetic variation associated with risk of CCA development? GAC CTG G GC C CG Adenine (A) – Thymine (T) Cytosine (C) – Guanine (G) Single nucleotide polymorphism (SNP) Is genetic variation associated with risk of CCA development? Blood DNA (N = 370) A * Blood DNA (N = 740) G * Single nucleotide polymorphism Genetic Variation in COX-2 Gene is Associated with CCA Risk % Increases in Risk 300% 50% rs2143417 Manuscript, submitted 40% rs689466 both Epidemiologic study • Current classification of CCA • Clinical risk factors for CCA • Genetic risk factors for CCA • Planned GWAS for CCA • Future directions Genome Wide Association Study (GWAS) GWAS for CCA Blood DNA (N = 2000) ** ** *** *** Blood DNA (N = 4000) ** ** *** *** Accrual for Phase I (n=1974) Alberta Health Services (44) University Health Network (62) Imperial College, UK (140) Biodonostia Research Institute, Spain (31) MD Anderson Cancer Center (739) Mayo Clinic Rochester (728) Mayo Clinic Arizona (200) Mayo Clinic Florida (12) University of California, San Francisco (18) National Cancer of Institute Future Directions of Genetic Risk Studies in CCA GWAS Discovery phase: Complete accrual, Grant application Validation phase: Begin accrual 2014 2015 2016 Genetic risk study in young-onset CCA Proportion of CCA patients aged < 50 18.4% iCCA GWAS Validation phase: Genotyping 2017 2018 Whole exome sequencing 17.3% pCCA 11.5% dCCA Summary • Genetic susceptibility for CCA remains poorly understood • Findings from GWAS of CCA will improve our understanding of • genetic predisposition • pathogenesis • New information will support efforts at prevention, diagnosis and treatment Clinical Outcomes study: Developing a New Clinical Staging System for pCCA Tumor Biology Clinical staging system Epidemiologic study Cholangio carcinoma Early diagnosis Personalized oncology Novel therapeutics ©2013 MFMER | 3299636-27 Stage I Single mass ≤ 3 cm Stage II Single mass ≤ 3 cm Vascular encasement Stage III Mass > 3 cm Intrahepatic and/or lymph node metastasis Stage IV Peritoneal metastasis Survival of pCCA Patients Classified by the New Staging System Survival (%) 100 P<0.0001 75 Stage I: 45.7 months (n=57) 50 Stage II: 13.8 months (n=89) 25 Stage III: 8.0 months (n=79) Stage IV: 2.1 months (n=38) 0 1 Manuscript, submitted 2 Years 3 4 5 ©2013 MFMER | 3299636-32 The International Hepatobiliary Neoplasia Biorepository Collects Liver Tissues Questionnaire Blood DNA plasma & serum Tumor & Benign tissue Clinical data Urine, Stool & Bile Next Generation Sequencing * ** Key driver mutations in CCA genome Cancer tissue are identified Candidate targeted drugs are tested in mice Implant into mice Best candidate drug is used for clinical therapy of the patient Complete response Partial response No response Summary of Current Projects in the International Hepatobiliary Neoplasia Biorepository Novel gene mutations Clinical staging system Patientderived xenograft mouse model Genetic markers and biomarkers Cholangio carcinoma Clinical & genetic risk factors Targeted therapies ©2013 MFMER | 3299636-35 Acknowledgements • Mayo Genome Consortia • Dr. Manal Hasan, MD Anderson Cancer Center • Dr. Mitesh J. Borad, Mayo Clinic, Scottdale, ARZ • Dr. Tushar C. Patel, Mayo Clinic, Jacksonville, FL • Dr. R. Kate (Katie) Kelley, University of California San Francisco • Dr. Oliver Bathe, Alberta Health Service, Canada • Dr. Sean Kelly, University Health Network, Canada • Dr. Shahid Khan, Imperial College, UK • Dr. Jesus Banales, Biodonostia Research Institute, Spain • All CCA patients and family members