Antibiotic Update
advertisement

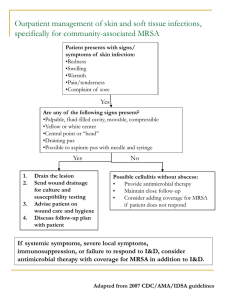
Review and Update of Antibacterial Drug Therapy Daniel Streetman, PharmD, MS Pharmacotherapy Specialist Lexicomp | Wolters Kluwer Health Objectives • Describe factors to consider when prescribing antibiotics • Compare some of the antibiotic classes used to treat common infections among community-dwelling individuals • Discuss the clinical application of this information for specific types of infections Antimicrobial Selection Systematic Process • Confirm infection • Identify pathogen(s) • Begin presumptive therapy • Monitor Antimicrobial Selection Systematic Process • Confirm infection • Identify pathogen(s) • Begin presumptive therapy • Monitor • Decreased antimicrobial use particularly of broad-spectrum agents • Less resistance, cost, toxicity Risks of Antimicrobial Use Resistance and Toxicity • Limited pipeline • CDC "Urgent Threats" – C. difficile – Carbapenem-resistant Enterobacteriaceae – N. gonorrheae http://www.cdc.gov/drugresistance/threatreport-2013/pdf/ar-threats-2013-508.pdf Cochrane Database Syst Rev 2013 Jan 31. Risks of Antimicrobial Use Resistance and Toxicity • Review of 11 RCTs, >3500 AOM episodes • Per 100 abx tx's: • CDC "Urgent Threats" – C. difficile – Carbapenem-resistant Enterobacteriaceae – N. gonorrheae – – – – – – 5 fewer w/ pain at 2-3 d 3 fewer perforations 9 fewer infx of o/ear no diff in other outcomes no diff in future AOM risk 7 toxicities (V/D, rash) http://www.cdc.gov/drugresistance/threatreport-2013/pdf/ar-threats-2013-508.pdf Cochrane Database Syst Rev 2013 Jan 31. Antibiotics Are Common Cause of ADE-Related ER Visits Clin Infect Dis 2008;47:735-43. Antimicrobial Selection Systematic Process • Confirm infection • Identify pathogen(s) • Begin presumptive therapy • Monitor Confirm Infection and Identify Pathogen • Fever, Leukocytosis, Local signs/symptoms – Drug-induced fever – Antipyretic use – Steroid-induced leukocytosis • Viral vs. Bacterial vs. Other – Sample infected tissue (Gm-stain, culture, etc.) – Contamination vs. Infection – Suspected pathogen(s) for specific site Acute Otitis Media: S.pneumoniae H.influenzae M.catarrhalis Viruses UTI: E.coli (85%) S.saprophyticus Enterococcus spp. K.pneumoniae P.aeruginosa Proteus spp. Enterobacter spp. SSTI: S .aureus S.pyogenes S.agalactiae Pharyngitis: Viruses S.pyogenes CABP: S.pneumoniae H.influenzae M.catarrhalis M.pneumoniae C.pneumoniae L.pneumophila Viruses Aspiration Pneumonia: Oral anaerobes S.viridans Enteric gm(-) bacilli Hospital-Acquired: S.aureus (MRSA) ESBL gm(-)s Antimicrobial Selection Systematic Process • Confirm infection • Identify pathogen(s) • Begin presumptive therapy • Monitor Initiate Presumptive Therapy • β-lactams – – – – • • • • • Penicillins Cephalosporins Carbapenems Monobactams Macrolides Tetracyclines Fluoroquinolones Sulfonamides Aminoglycosides • • • • • • • • • Vancomycin Clindamycin Metronidazole Linezolid Quinupristin/Dalfopristin Daptomycin Telavancin Rifamycins Urinary antiseptics Initiate Presumptive Therapy Patient Factors • • • • • • Severity and acuity Allergies Age Comorbidities (including pregnancy) Genetics Concurrent medications Initiate Presumptive Therapy Allergy • Is this rash an allergy? – "Ampicillin rash" • up to 80-100% of pts with mononucleosis • 33% of amoxicillin recipients vs. 23% non-amox • cefalexin, cefaclor, cefadroxil most closely related – 72% tolerated these vs. 97% of other cephalosporins – Post-viral rash – Streptococcal rash Pediatrics 2013;131(5):e1424-7. J Antimicrob Chemother 2007;60(1):107-11. Initiate Presumptive Therapy Allergy • 80-90% of those with reported allergy to PCN have negative skin test – 97-99% can receive PCN without immediate-type hypersensitivity reaction Mayo Clin Proc 2005;80:405-10. N Engl J Med 2001;345:804-9. Monitoring Renal Function for Drug Therapy • Est Creatinine Clearance = [140-age(yrs)] Weight (kg) (Serum Creatinine 72) <Note: multiply above result by 0.85 for females!> • This often overestimates GFR in older patients! Creatinine Clearance (mL/min) • Glomerular filtration is likely the most sensitive to agerelated change (vs. secretion or reabsorption) 140 120 100 80 60 40 20 Renal blood flow s from 120 mL/min at 30-40 years of age to 60 mL/min at 80 years of age. 0 30s 40s 50s 60s 70s 80s Age (Decade) Initiate Presumptive Therapy Age Caution in children: • Tetracyclines • Chloramphenicol Caution in older pts: • β-lactams, vanco, etc. • Fluoroquinolones • Isoniazid Incidence (%) Tetracycline tooth staining 9 8 7 6 5 4 3 2 1 0 21-35 35-49 50-64 65+ Patient Age (yrs) Incidence of INH Hepatotoxicity Initiate Presumptive Therapy Comorbidities • Renal, hepatic disease • Cystic fibrosis, Diabetes, Burn patients, Neutropenic patients, HIV/AIDS, etc. • Specific toxicity-related concerns – Ticarcillin, piperacillin: high Na+ content – Sulfonamides: crystalluria – Fluoroquinolones: myasthenia gravis Initiate Presumptive Therapy Concurrent Medications • Macrolides: inhibit CYP3A4 • Fluoroquinolones: inhibit CYP1A2; binding to Al3+, Mg3+, Ca2+, Fe3+ • Tetracyclines: binding Al3+, Mg3+, Ca2+, Fe3+ • Linezolid: MAO inhibition • β-lactams: increased conc's with probenecid • Rifampin: major enzyme inducer Initiate Presumptive Therapy Drug Factors • Local sensitivities/recommendations • Pharmacodynamics • Pharmacokinetics – Route – Distribution – Interactions • Toxicities • Cost Antibiotics Local Sensitivities and Recommendations • SST: treat for 7-10 days (PO) or 10-14+ days (IV/PO) – If CA-MRSA is not a concern: dicloxacillin or cephalexin – If CA-MRSA is concern: clindamycin, doxycycline, or SMZ/TMP (± dicloxacillin or cephalexin) Concern for MRSA increases with: Abscesses, Exudative lesions, Community prevalence of > 15% Initiate Presumptive Therapy Pharmacodynamics - Mechanism(s) of Action • Most abx work by only few general mechanisms: – Disrupt bacterial cell wall • Beta-lactams, Vancomycin – Interfere with bacterial protein/DNA/RNA synthesis • Macrolides/Azalides/Ketolides, Tetracyclines, Aminoglycosides, Clindamycin, Linezolid, Quinupristin/Dalfopristin – Block bacterial folic acid synthesis • Sulfonamides, Trimethoprim – Disrupt DNA transcription/translation • Fluoroquinolones – Other • Daptomycin, Metronidazole, most anti-TB drugs Initiate Presumptive Therapy Pharmacodynamics - "cidal" vs. "static" • Antibacterials that actually kill the bacteria in the body are classified as "bactericidal" – kill at least 99.9% of bacterial population – less than 3-log reduction = "bacteriostatic" • Most drugs that inhibit protein synthesis are only bacteriostatic (exception: aminoglycosides) – Other "cidal" drugs include beta-lactams, vancomycin, fluoroquinolones Initiate Presumptive Therapy Pharmacodynamics - Optimal dosing • Beta-lactams – Time > MIC • Aminoglycosides – Peak:MIC • Fluoroquinolones Initiate Presumptive Therapy Pharmacokinetics • Route of administration – Low/no oral bioavailability • Vancomycin, Rifaximin, Fidaxomicin – High/consistent oral bioavailability • Fluoroquinolones, Linezolid • Distribution – Macrolides, Fluoroquinolones, Tetracyclines with activity vs. Mycoplasma pneumoniae, Legionella pneumophila, Chlamydia pneumoniae Initiate Presumptive Therapy Drug Factors • Local sensitivities/recommendations • Pharmacodynamics • Pharmacokinetics – Route – Distribution – Interactions • Toxicities • Cost Antimicrobial Selection Systematic Process • Confirm infection • Identify pathogen(s) • Begin presumptive therapy • Monitor Monitor Therapy • Fever, WBC, Local signs and symptoms • Need for changing therapy – Failure – Streamlining, IV to PO • Antimicrobial serum concentrations • Toxicity-related testing Monitor Therapy Recommended Testing • Antimicrobial serum concentrations – Aminoglycosides – Vancomycin – Chloramphenicol • Toxicity-related testing – Renal function, hydration status Monitor Therapy Failure • • • • Inadequate diagnosis Poor initial drug selection Poor source control New infection – Resistant population – Secondary infection Supplemental Information and Case Discussions CABP Pathogens and Guidelines • Likely pathogens: – S. pneumoniae, H. influenzae, M. catarrhalis, M. pneumoniae, C. pneumoniae, L. pneumophila, viruses • CABP: macrolide, doxycycline, respiratory quinolone, or β-lactam+macrolide* – ≥ 5 days, depending on clinical picture – 5 days: azithromycin or levofloxacin (750 mg dose) – 7-10 days: other oral agents *Only if bacterial ... 20-25% of abx use 'inappropriate' Macrolides • Erythromycin, Azithromycin (Zithromax), Dirithromycin (Dynabac), Clarithromycin (Biaxin) • Inhibit protein synthesis – Bind to 50S ribosomal subunit – Usually bacteriostatic, but can be bactericidal • Spectrum: Gram + (staph, strep); Atypicals (Mycoplasma, Chlamydia, Legionella) • Substrates and inhibitors of CYP3A4, Pgp – Azithromycin has unique kinetics Macrolides • Abdominal pain, N/V/D • QTc prolongation • May increase GI motility ... motilin agonist – specific to erythromycin and azithromycin Macrolide Drug Interaction Concerns • Moderate to Strong CYP3A4 inhibitors – Steroids, CCBs, statins, BZDs, AEDs, more • Inhibit OATP1B1 – Increase pravastatin AUC 2.1-fold, other statins by up to 12-fold • Inhibit P-glycoprotein – P-glycoprotein, newer anticoagulants Macrolides May Increase Risk of Cardiac-Related Death • Erythromycin known to prolong QT interval – Also inhibitor of CYP3A, OATP1B1, and p-glycoprotein – >2-fold increase in SCD with eryth vs. o/abx – >5-fold increase with eryth and CYP3A inhibitor • Clarithromycin also seems to share similar risks (QT effects, CYP3A, p-gp, etc.) N Engl J Med 2004;351:1089-96. BMJ 2013;346:f1235. Does Azithromycin Increase Risk of Cardiac-Related Deaths? N Engl J Med 2013;368:1704-12. N Engl J Med 2012;366:1881-90. Tetracyclines • Tetracycline, doxycycline (Vibramycin), minocycline (Minocin), tigecycline (Tygacil) • Inhibit protein synthesis – Inhibit 30S ribosomal subunit ... bacteriostatic • Broad spectrum agents, including atypicals, H.pylori, Propionibacterium acnes – Including MRSA • Variable lipid solubility and half-life (6 to >24 hrs) – TCN = 6-8 hrs – minocycline, doxycycline, tigecycline = ≥ 16 hrs Tetracyclines • Interactions: divalent chelation (GI interactions) • GI burning, cramps, N/V/D • Tooth discoloration, suppressed long bone growth – Avoid in later pregnancy and in children < 8 yrs of age • Photosensitivity, hepatotoxicity – Special caution with expired meds Interactions with Tetracyclines and Fluoroquinolones • 84% in doxycycline AUC with Al3+/Mg3+based antacid • ≤ 51% in doxy and TCN absorption with bismuth • FQs also inhibit CYP1A2 Bioavailability (%) Al3+/Mg3+ Ca2+ 100 90 80 70 60 50 40 30 20 10 0 ciproflox levoflox norflox 45-97% with Al3+/Mg3+ 3-63% with Ca2+ Fluoroquinolones • Ciprofloxacin (Cipro), Levofloxacin (Levaquin), Norfloxacin (Noroxin), Ofloxacin (Floxin), Lomefloxacin (Maxaquin), Sparfloxacin (Zagam), Moxifloxacin (Avelox), Gemifloxacin (Factive) • Inhibits DNA gyrase (topoisomerase II) and toposiomerase IV; required for DNA uncoiling during replication and cell division – Bactericidal • Active against many Gm(-) aerobes; many have good activity vs. many Gm(+) aerobes Fluoroquinolones • By 'generation' – 1st: nalidixic acid – 2nd: Ciprofloxacin (Cipro), Levofloxacin (Levaquin), Norfloxacin (Noroxin), Ofloxacin (Floxin) – 3rd: Gemifloxacin (Factive) – 4th: Moxifloxacin (Avelox) • "Respiratory" or not – "Respiratory" quinolone: levofloxacin, moxifloxacin, gemifloxacin – Ophthalmic: gatifloxacin, besifloxacin, ciprofloxacin, levofloxacin, moxifloxacin, ofloxacin Fluoroquinolones • Nearly 100% bioavailable (*chelation issues); hepatic metabolism, renal excretion • Resistance: altered binding target and/or efflux mechanisms (high- vs. low-level); low frequency – Increased use frequently cause, thus need to restrict – Animal feed • Polyvalent cations, CYP1A2 substrates • GI effects, QTc prolongation, hyper/hypoglycemia, arthropathy and tendonitis (limits pediatric use), seizures Fluoroquinolone Toxicities • Neuropsychiatric effects – CNS stimulation • Tendon rupture – Age > 60 yrs – Steroid use – Post-transplant • QT prolongation • Hyper-/hypoglycemia AOM Pathogens and Guidelines • S. pneumoniae, H. influenzae, M. catarrhalis, viruses • OM: amoxicillin (or amox/clavulanic acid or clindamycin or cephalosporin)** – Cephalosporins = cefuroxime, cefpodoxime, cefdinir, ceftriaxone (IV/IM) – Alternatives: macrolide, sulfamethoxazole/trimethoprim – < 2 yrs old = 10 days – < 6 yrs old = 7-10 days – > 6 yrs old = 5-7 days **Only recommended if bilateral, severe presentation, or failure to improve after 48-72 hrs of "watchful waiting" Pharyngitis Pathogens and Guidelines • Viral, S. pyogenes (20-30% kids, 5-15% adults) • Pharyngitis: PCN VK, amoxicillin, or cephalosporin (or clindamycin)* – 10 days *Only if Strep-positive ... otherwise, likely viral • Amoxicillin higher-dose, given once daily is becoming preferred dose • Treatment decreases infectious period from 10 days to approx 24 hrs, and decreases symptoms by 1-2 days Beta-Lactams Penicillins • Block cross-linking of bacterial cell wall by endopeptidases (“PBPs”) – on interior of cell wall • Time-dependent killing • Resistance – Beta-lactamases (H.flu) – Alteration of PBPs (MRSA) Beta-Lactams Penicillin “Classes” • Natural penicillins – Penicillin • Extended-spectrum – Ampicillin, amoxicillin • Antistaphylococcal penicillins (ß-lac resistant) – Methicillin, nafcillin, oxacillin, dicloxacillin • Antipseudomonal penicillins – Piperacillin, ticarcillin Penicillins Notable Penicillins • Amoxicillin vs. Ampicillin – Amoxicillin/Clavulanic acid (Augmentin) – Ampicillin/Sulbactam (Unasyn) – May cause non-allergic rash • Piperacillin vs. Ticarcillin – Piperacillin/Tazobactam (Zosyn) – Ticarcillin/Sulbactam (Timentin) – HIGH sodium content Beta-Lactams Cephalosporins • Block cross-linking of bacterial cell wall by endopeptidases (“PBPs”) – on interior of cell wall • Resistance – Beta-lactamases – Alteration of PBPs Beta-Lactams Cephalosporins • Organized into "generations" based on spectrum and year introduced • Generally, with each generation: – Increased gm(-) and anaerobic activity – Greater resistance to -lactamase – Increased penetration of CNS • Mostly renally eliminated – Ceftriaxone has hepatic/biliary elimination Beta-Lactams Cephalosporins • First Generation – cefazolin*, cephalexin, cefadroxil • Second Generation – cefuroxime, cefoxitin*, cefotetan*, cefprozil, cefaclor • Third Generation – ceftazidime*, ceftriaxone*, cefotaxime*, cefixime, ceftibuten, cefdinir, cefditoren, cefpodoxime • Fourth Generation: cefepime* • Fifth Generation: ceftaroline* *Available as injectable product (IV and/or IM) Amoxicillin in AOM • 80-90 mg/kg/day dosing is preferred over conventional 40-45 mg/kg/day – Effective vs. PRSP • Alternatives only for treatment failure or allergy – Amoxicillin/clavulanate (prefer 14:1 ratio) – Cephalosporins – Macrolide, Clindamycin, SMZ/TMP Cost Comparison Pharyngitis Treatment Options UTI • UTI (lower): SMZ/TMP, nitrofurantoin, quinolone Uncomplicated – SMZ/TMP or FQ = 3 days – nitrofurantoin = 5 days – amoxicillin/clavulanate = 3 days Complicated – SMZ/TMP or FQ = 7-10 days – amoxicillin/clavulanate = 7-10 days • UTI (upper): SMZ/TMP, ciproflox, levoflox – SMZ/TMP = 14 days – ciprofloxacin = 7-14 days – levofloxacin = 5-14 days SSTI Pathogens and Guidelines • S. aureus, S. pyogenes, S. agalactiae • SST: treat for 7-10 days (PO) or 10-14+ days (IV/PO) – If CA-MRSA is not a concern: dicloxacillin or cephalexin – If CA-MRSA is concern: clindamycin, doxycycline, or SMZ/TMP (± dicloxacillin or cephalexin) Other Unique Antibacterials • Telithromycin (Ketek) – Related to macrolides; reserve for MDRSP – Serious liver toxicity risks; drug interaction risk • Linezolid (Zyvox) – Active vs. VRE, MRSA – Weak MAO inhibition … interaction risks! • Quinupristin/Dalfopristin (Synercid) – Active vs. VRE, MRSA, MRSE, MDRSP – Hepatotoxicity, phlebitis/local pain, arthralgia/myalgia • Daptomycin (Cubicin) – Unique MOA (depolarizes cell membrane) – Active vs. MRSA – Myopathy, neuropathy Antibiotics Guidelines for Common Infections • CABP: macrolide, doxycycline, respiratory quinolone, or β-lactam+macrolide* – ≥ 5 days, depending on clinical picture *Only if bacterial ... 20-25% of abx use 'inappropriate' • OM: amoxicillin (or amox/clavulanic acid or clindamycin or cephalosporin)** – < 2 yrs old = 10 days – < 6 yrs old = 7-10 days – > 6 yrs old = 5-7 days **Only recommended if bilateral, severe presentation, or failure to improve after 48-72 hrs of "watchful waiting" Antibiotics Guidelines for Common Infections • Pharyngitis: PCN VK, amoxicillin, or cephalosporin (or clindamycin)* – 10 days *Only if Strep-positive ... otherwise, likely viral • SST: treat for 7-10 days (PO) or 10-14+ days (IV/PO) – If CA-MRSA is not a concern: dicloxacillin or cephalexin – If CA-MRSA is concern: clindamycin, doxycycline, or SMZ/TMP (± dicloxacillin or cephalexin) • UTI: SMZ/TMP, nitrofurantoin, quinolone – SMZ/TMP or FQ = 3 days – nitrofurantoin = 5 days Spectrum of Activity • Drugs vary widely regarding spectrum of activity, and detailed knowledge of this will require much study and/or experience – Even within same class, spectrum can be quite different • A few general notes about spectrum for each group of drugs follows in class-specific discussions ... Methods of Resistance • Inactivating enzymes – -lactamase, etc. • Alteration of drug target – Changes in 50S, 30S subunits – Mutation in DNA gyrase – Altered penicillin binding proteins • Expression of drug efflux transporter – TCNs, macrolides, fluoroquinolones More About Resistance • Transferrable – Person-to-person – Bacteria-to-bacteria – Plasmid-to-plasmid plasmid-to-chromosome • Strongly influenced by antibiotic use – Lower concentrations – Incomplete courses • Increasingly limited antibiotic pipeline – Most current abx discovered pre-1970 Antibiotics Cephalosporins Adverse Effects • Allergy - cross reaction up to 10% w/PCN – 1-2% w/o PCN allergy • CNS - drug fever, seizures • Hematologic – Hemolytic anemia, rare bone marrow suppression – N-methylthiotetrazole (NMTT) side chain: interferes with vitamin-K dependent coagulation factor synthesis & possible disulfiram reaction ... cefotetan • Diarrhea and C.difficile colitis • Interstitial nephritis Beta-Lactams Carbapenems, Monobactams • Imipenem, meropenem, ertapenem, doripenem – – – – Severe polymicrobial infections, very broad spectrum Cross-reactive with penicillins/cephalosporins Cilastatin = dipeptidase inhibitor (used w/imipenem) Seizure risk • Aztreonam – – – – Limited to gram negative rods May include Pseudomonas Occasionally used as alternative to AG No cross-allergy to PCNs • Concern with ceftazidime Vancomycin • Inhibits bacterial peptidoglycan production – Binds D-ala-D-ala component of peptidoglycan – Only effective vs. gm(+) organisms • Critical "last resort" medication – Emerging resistance a concern • Kinetics: – No oral absorption – ~90% renal elimination • Phlebitis, “Red Man” syndrome, nephrotoxicity, ototoxicity Aminoglycosides • Gentamicin, tobramycin, amikacin • Inhibition of protein synthesis, altered protein synthesis (due to misreading) – binds to 30S ribosomal subunit – bactericidal (concentration-dependent) • Spectrum: mostly aerobic Gm(-) – Syngery with ß-lactams (conc.-dependent instability) • Renal excretion – Highly variable elimination – Can use serum concentrations to guide dosing Aminoglycosides • Nephrotoxicity – High trough concentrations (Cmin > 2) – Cumulative exposure, elderly, other nephrotoxic drugs • Ototoxicity, neuromuscular blockade (high dose) Sulfonamides • Sulfamethoxazole, others • Inhibits bacterial folic acid synthesis – p-aminobenzoic acid (PABA) analog that competes as substrate for folic acid synthesis (required for DNA synthesis) – Often given with trimethoprim (inhibitor of folic acid activation) to achieve synergy • Broad spectrum (including MRSA) – Pneumocystis jiroveci (P. carinii) Sulfonamides • Hepatic metabolism (acetylation), mostly renal excretion • Interactions: warfarin, sulfonylureas • ADRs: allergy (cross-sensitive to other “sulfas”) – – – – – Can precipitate in acidic urine (drink water) Hemolytic anemia (G6PD) Photosensitivity Severe skin reactions (SJS, TENs) Megaloblastic anemia (rare) Other Unique Antibacterials • Telithromycin (Ketek) – Related to macrolides; reserve for MDRSP – Serious liver toxicity risks; drug interaction risk • Linezolid (Zyvox) – Active vs. VRE, MRSA – Weak MAO inhibition … interaction risks! • Quinupristin/Dalfopristin (Synercid) – Active vs. VRE, MRSA, MRSE, MDRSP – Hepatotoxicity, phlebitis/local pain, arthralgia/myalgia • Daptomycin (Cubicin) – Unique MOA (depolarizes cell membrane) – Active vs. MRSA – Myopathy, neuropathy Clindamycin (Cleocin) • Inhibits protein synthesis (binds 50S) • Spectrum: most anaerobes (except C. difficile), Gm(+) aerobes – Active against MRSA • Widely distributed (except CNS) • Largely metabolized, mixed elimination • Diarrhea, pseudomembranous colitis • Hepatotoxicity, rashes, blood dyscrasias Metronidazole (Flagyl) • Classified as antiprotozoal • Spectrum: anaerobes including Bacteroides and Clostridium • MOA: – Accepts electrons (deprives fermentation chemistry) – Reduced molecule toxic to DNA • Mixed anaerobic and colitis (GI), also CNS (abscess) • ADR: disulfiram effect Rifaximin (Xifaxan) • Rifamycin antibiotic indicated for (1) traveler’s diarrhea due to E. coli and (2) hepatic encephalopathy – Use for C. difficile-associated diarrhea (CDAD) is an unlabeled use (treatment, "chaser") – Inhibits RNA synthesis • 200mg and 550mg tablets; given BID-TID • Limited systemic absorption – Low side effect, interaction potential Fidaxomicin (Dificid) • Macrolide antibiotic indicated for treatment of C. difficile-associated diarrhea – Inhibits RNA synthesis (bactericidal) • Available as 200 mg tablets, given BID • Minimal systemic absorption (<10%) in healthy volunteers – Appears to be higher (2- to 6-fold) in patients