Work Stream 2: Regulatory Environment Lead
advertisement

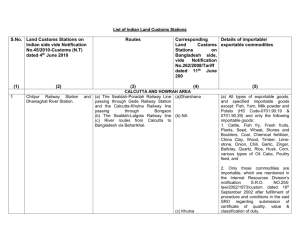
Quality, affordable drugs supplied in a sustained manner Causes under5 deaths iCCM of malaria, diarrhea, pneumonia Quality, affordable drugs supplied in a sustained manner Quality, affordable drugs supplied in a sustained manner Quality, affordable drugs supplied in a sustained manner Chlorhexidine End to end Injectable antibitiotics, amoxcillne ORS, zinc Learn lessons from successfull commodities such as vaccines, malaria commodities Quality, affordable drugs supplied in a sustained manner Private sector pharma Chlorhexidine Commission ? Norway $$$$ End to end Injectable antibitiotics, amoxcillne High level taskforce UN SG USAID ORS, zinc Quality, affordable drugs supplied in a sustained manner Work on market shaping End to end Commission Work on incountry bottlenecks AU G8 Work on market shaping End to end Quality, affordable drugs supplied in a sustained manner Work on incountry bottlenecks Causes under5 deaths Supply and demand/ careseeking The United Nations Commission on Life-Saving Commodities for Women and Children • • Visit the website of the Commission: • http://www.everywomaneverychild.org/resou rces/un-commission-on-life-savingcommodities THE COMMISSION • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • • Co-Chairs: HE Mr. Jens Stoltenberg, Prime Minister of Norway HE Mr. Goodluck Ebele Jonathan, GCON, President of the Federal Republic of Nigeria Vice Chairs: Tony Lake, Executive Director, UNICEF Dr. Babatunde Osotimehin, Executive Director, UNFPA Technical Working Group Work Stream Leads: Market Shaping: Oliver Sabot, Executive Vice President for Global Programs at the Clinton Health Access Initiative (CHAI), Regulatory: Hans Hogerzeil, Former Director for Essential Medicines and Pharmaceutical Policies, WHO and Professor of Global Health, Groningen University Best Practices and Innovation: Patricia Mechael, Executive Director, mHealth Alliance; and Catharine Taylor, Director of the Maternal and Child Health and Nutrition Program, PATH Commissioners: Sir Andrew Witty, Chief Executive Officer, GlaxoSmithKline Agnès Saint-Raymond, Head of Human Medicines Special Areas, European Medicines Agency Bob Collymore, Chief Executive Officer, Safaricom Christopher Elias, President for Global Development, Bill and Melinda Gates Foundation Dan Brutto, President, UPS International Gary Cohen, Executive Vice President, Becton Dickson (BD) Hassan Mshinda, Director General Tanzania Commission for Science and Technology Heather Bresch, CEO , Mylan Inc Jamie Cooper-Hohn, President and CEO, Children's Investment Fund Foundation Jasmine Whitbread, Chief Executive Officer, Save the Children International Julio Frenk, Chair, Partnership for Maternal, Newborn and Child Health Kenneth C. Frazier, President and CEO, Merck Rajiv Shah, Administrator, United States Agency for International Development Ray Chambers, UN Secretary-General's Special Envoy for Malaria Li Dongjiu (Robert Lee), President, Shanghai Fosun Pharmaceutical Development Co., Ltd. M.K. Bhan, Secretary to the Government of India Department of Biotechnology Michael Anderson, Director-General for Policy and Global Programmes, UK Department for International Development Per Heggenes, CEO, IKEA Foundation Teguest Guerma, Director General, AMREF H.E. Zainab Hawa Bangura, Minister of Health and Sanitation, Sierra Leone LIFE-SAVING COMMODITIES RMNCH Continuum of Care Reproductive health Commodity Usage Female Condoms Family planning/ Contraception Implants Family planning/ Contraception Emergency Contraception Family planning/ Contraception Oxytocin Post-Partum Hemorrhage Misoprostol Post-Partum Hemorrhage Magnesium sulfate Eclampsia and Severe Pre-Eclamsia/Toxemia of Pregnancy Injectable antibiotics Newborn Sepsis Chlorhexidine Newborn Cord Care Resuscitation Equipment Newborn Asphyxia Antenatal Corticosteroid (ANCS) Respiratory Distress Syndrome for preterm babies Amoxicillin Pneumonia Oral Rehydration Salts (ORS) Diarrhea Zinc Diarrhea Maternal Health Newborn Health Child Health • All product profiles and case studies are available on the Commission Website: • http://www.everywomaneverychild.org/resou rces/un-commission-on-life-savingcommodities/life-saving-commodities • TECHNICAL WORKING GROUP Work • Work Stream 1: Market Shaping • Work Stream 2: Regulatory Environment • Work Stream 3: Best Practices and Innovations TIMELINE • Key upcoming milestones, events and advocacy opportunities: – May 15 - Commissioners receive draft recommendations and materials for 22 May meeting – May 21-26 - World Health Assembly – distribution of information on the Commission and side event (TBC) – May 22nd - 2nd Commissioners meeting in New York – June 14th -15th - Child Survival Forum – discussion of draft recommendations at Commission focused event – July 12th- Family Planning Summit – July 27th -28th – iERG’s draft annual report – August 1st- Launch of Global Health Policy Forum – August- Launch of A Promise to Keep – September- UN General Assembly- Launch of final report of the Commission at the Every Woman Every Child event – October- PMNCH Board Meeting in Nigeria – Executive Board meetings of UNFPA and UNICEF; and African Union meetings. • TECHNICAL WORKING GROUP Work Stream 1: Market Shaping • • • • • • • Lead: Oliver Sabot, Executive Vice President for Global Programs at the Clinton Health Access Initiative (CHAI) Key issue: One key challenge affecting many of the commodities is insufficient market dynamic; for example on the supply side, a lack of manufacturers producing commodities, or in regards to demand, the lack of knowledge around the availability of commodities. This group will therefore look into market shaping opportunities. This includes strategies and implementation plans to create an enabling environment that helps ensure an appropriate supply of quality and affordable products. This work stream will explore opportunities in creating such an enabling environment for the 13 identified commodities through changes and developments in health policy, innovative financing mechanisms, supply chain strengthening, and regulatory approaches. Key next steps: Develop an overall framework to diagnose and categorize market problems and propose specific interventions to address the market issues Create a comprehensive tool-kit that can be used to address bottlenecks and market problems. Test the toolkit on 2 or 3 sample products from the Commission list of 13 to determine validity of the framework and toolkit. Provide the specific recommendations based on results of analysis. Work Stream 2: Regulatory Environment • • • • • • • Lead: Hans Hogerzeil, Former Director for Essential Medicines and Pharmaceutical Policies, WHO and Professor of Global Health, Groningen University Key issue: Critical bottlenecks affecting the commodities are also found in the regulatory environment, which is shaped by the requirements of National Medicine Regulatory Authorities (NMRA). These typically have oversight in three essential areas of making a quality medicine available in the marketplace: assuring the quality and efficacy of medicines; providing necessary authorizations for distribution of the medicine; and supporting eligibility for procurement with public funding. Each country has unique requirements that manufacturers must meet, including separate, detailed dossiers and possibly physical inspections of the manufacturing facility. Approval from the WHO prequalification system or another recognized stringent regulatory authority is also required for publicly funded procurement. Regulatory processes are a critical value-added function, but they represent significant investments, and sometime have the unintended effect of limiting the participation of smaller manufacturers. Based on review of the case studies the following regulatory challenges were identified Registration of new dosing/ formulas Changing classifications to Over the Counter (OTC) Changes related to WHO’s Essential Medicines List Poor matching between disease burden and scalability of regulatory changes Work Stream 2: Regulatory Environment • Key next steps: • Continue discussions and collaboration with WHO, Paediatric medicines Regulators' Network (PmRN) (includes members from 26 countries), national and regional regulatory authorities, as well as with Commissioner Ms Agnès Saint Raymond. • Identify short vs. long term interventions and recommendations that include • i) Simplification of regulatory procedures such as introduction of one, simple file for regulators that help them make a proper but more rapid risk assessment to ensure rapid uptake of low-risk commodities; and • ii) Identification and sharing of best practices with regards to OTC status. Work Stream 3: Best Practices and Innovations • Leads: Patricia Mechael, Executive Director of the mHealth Alliance and Catharine H. Taylor, Catharine Taylor the Director of the Maternal and Child Health and Nutrition Program at PATH • Key issue: This stream of work will focus on the following areas that show potential for rapidly increasing access to and use of the commodities, building on findings and recommendations from the Innovations Working Group of the UN Commission on Information and Accountability: Innovative Technologies that may facilitate access to a product, such as better medical devices, packaging, and formulations, as well as complementary technologies such as mobile phones or SMS. Strategies to Create Demand amongst healthcare professionals and/or patients/caregivers, such as better communications, training, user fee waivers, cash transfers, or insurance schemes. Innovations for Scaling Up access to a given commodity at a local level, based on a systematic analysis of existing interventions to overcome bottlenecks in public sector, private sector, and at community level. Key next steps Enhance linkages to the Innovations Working Group of the Every Woman Every Child movement; Synthesise existing innovative approaches to provide context including scope, and identification of gaps and opportunities and gaps. • • • • • • TIMELINE • Key upcoming milestones, events and advocacy opportunities: – May 15 - Commissioners receive draft recommendations and materials for 22 May meeting – May 21-26 - World Health Assembly – distribution of information on the Commission and side event (TBC) – May 22nd - 2nd Commissioners meeting in New York – June 14th -15th - Child Survival Forum – discussion of draft recommendations at Commission focused event – July 12th- Family Planning Summit – July 27th -28th – iERG’s draft annual report – August 1st- Launch of Global Health Policy Forum – August- Launch of A Promise to Keep – September- UN General Assembly- Launch of final report of the Commission at the Every Woman Every Child event – October- PMNCH Board Meeting in Nigeria – Executive Board meetings of UNFPA and UNICEF; and African Union meetings. •