Humidification - Respiratory Therapy Files
advertisement

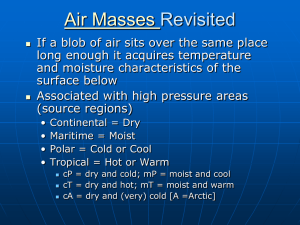
RT Physics Week 4 • • • • QUIZ Review of Pressure Conversions Humidity and Aerosol Review – PAO2 – P(A-a) – CaO2 – DO2 Pressure Conversions • Respiratory therapists use several different pressure measurement scales. We have already discussed conversion from gauge to absolute pressure. It is also important to be able to convert from one scale to another, ie: cmH2O to torr or psig to cmH2O. Pressure Conversions • One approach to converting from one pressure scale to another would be to memorize a conversion factor for each pressure scale. An easier approach is to remember the value for 1 atmosphere in each of the commonly used pressure scales. • Remember: 1 ATM = 15 psi = 760 torr = 1034 cmH2O Pressure Conversion • Then use the following equation to convert: • New Pressure= 1 ATM in new scale ------------------------- x Given pressure Value 1 ATM given scale Pressure Conversion • Example: Convert 590 torr to cmH2O • New Pressure value= 1034 cmH2O x 590 torr ----------------760 torr Torr units cancel New pressure value= 1.36 x 590 = 802 cmH2O Remember: 1 ATM = 15 psi = 760 torr = 1034 cmH2O Humidity and Aerosol • The body’s systems require a certain amount of hydration in order to maintain homeostasis. The best way is through drinking plenty of fluids and administration of IV fluids. Signs of dehydration are chapped lips, flaky skin, dry cracked elbows and heels. The respiratory signs include crusty nasal cavities, nosebleeds, dry mouth, scratchy throat and a dry hacking cough. Humidity and Aerosol • Many of our patients have bypassed normal airway and an important part of our job is to provide for their special needs. • Under normal conditions, body water loss through respiration is approximately 200 – 500 mL / day. Respiratory therapists deliver oxygen that needs to humidified and often heated to adequately meet the needs of their patients. Humidity and Aerosol • Vapor pressure – Pressure water as a vapor or gas exerts and is part of the total atmospheric pressure. Water vapor pressure in the lungs exert 47 mmHg • Absolute Humidity – the actual amount (in mg./l) of water vapor in the atmosphere • Relative Humidity – the percent of water vapor in the air as compared to the amount necessary to cause saturation at the same temperature. • % Body Humidity – the relative humidity at 37 degrees Celsius • Humidity Deficit – the amount of water vapor needed to achieve full saturation at body temperature (44 mg/l - A.H) Vapor pressure Vaporization: the change of matter from a liquid to a gaseous form Water vapor pressure – the direct measure of the kinetic activity of water vapor molecules Reducing the pressure above a liquid lowers its boiling point. Ex. water boiling in mountains Water Vapor Pressure • When a gas is in contact with a liquid, and is in equilibrium (saturated) with the liquid, the partial pressure of the gas is a function of temperature. The one gas to which this applies in a normal respiration is water. The lungs and airways are always moist, and inspired gas is rapidly saturated with water vapor in the upper segments of the respiratory system. The temperature in the airways and lungs is almost identical with deep body temperature (approximately 37°C); at this temperature water vapor has a partial pressure of 47 mmHg. (Note that the gaseous form of a liquid frequently is termed a "vapor"). • Using the value of 47 mmHg, we can calculate partial pressure of oxygen and nitrogen in inspired air, after the gas mixture becomes saturated with water vapor in the upper airway (so-called tracheal air): • Ptotal = 760 mmHg PH20 = 47 mmHg --713 mmHg for remaining inspired gases (21% O2 and 79% N2) • PO2 = 0.21 · 713 = 150 mmHg PN2 = 0.79 · 713 = 563 mmHg Water Vapor Pressure • That is, since water vapor partial pressure must be 47 mmHg in a saturated gas mixture at 37°C, the total pressure remaining for the inspired gases is only 760-47 or 713 mmHg. The composition of this remaining gas is 21% O2 and 79% N2, giving the partial pressures indicated above which is then substrated by the partial pressure of PaCO2 (PACO2, is a product of the amount of CO2 diffused into the lung) • PAO2 = FIO2 (Pb-PH2O) – (PaCO2/0.8) Humidification • Absolute humidity: the actual content or water vapor present in a given volume of air • Relative humidity: the actual water vapor present in a gas compared with the capacity of that gas to hold the vapor at a given temperature – If the water vapor content of a volume of gas equals its capacity, the relative humidity of the gas equals 100% Both are essential in effective ventilation. Prevents drying of airway mucosa and irritation. Various respiratory care devices are used to ensure adequate humidification of inspired gases. http://www.youtube.com/watch?v=CL5cgX wKUXc Humidity • The NOSE is the bodies natural humidifier and filter, when bypassed we must use a artificial humidifier Humidity Terms • Vapor pressure – Pressure water as a vapor or gas exerts and is part of the total atmospheric pressure. Water vapor pressure in the lungs exert 47 mmHg • Absolute Humidity – the actual amount (in mg./l) of water vapor in the atmosphere • Relative Humidity – the percent of water vapor in the air as compared to the amount necessary to cause saturation at the same temperature. • % Body Humidity – the relative humidity at 37 degrees Celsius • Humidity Deficit – the amount of water vapor needed to achieve full saturation at body temperature (44 mg/l - A.H) • Isothermic Saturation Boundary – At or just below carina (end of trachea) The point at which inspired gases are fully 100% saturated and warmed to body temperature (44 mg/L at 37oC) Humidity • • • • • • Uses of Humidity therapy Humidification of inspired gases Thinning of bronchial secretions Sputum induction Solutions Used • Sterile water used in humidifiers and continuous nebulizers (Hypotonic) • (Normal) Isotonic saline (.9% Na) with (Aerosol / Medicine) Treatments • Hypertonic saline (10%) (for sputum induction) Example 0 • A gas is flowing thru a ventilator circuit at 50 C with a relative humidity of 100%. As it flows thru the tubing it is cooled to 37 C by the surrounding ambient temperature0 of the room. What effects will occur within the tubing? What will occur to the ambient temperature of the air surrounding the tubing? 1. Condensation will occur on the inside surface of the tubing as the water vapor reaches its dew point 2. There will be visible droplet formation when dew point is reached 3. There will be warming of the adjacent air due to convection Humidity and Aerosol • Solutions Used • Sterile water used in humidifiers and continuous nebulizers (Hypotonic) • (Normal) Isotonic saline (.9% Na) with (Aerosol / Medicine) Treatments • Hypertonic saline (10%) (for sputum induction) Consequences of Inadequate Humidification • Inspissated secretions • Damage to tracheal epithelium • Decrease in ciliary activity • Retention of secretions Consequences of Inadequate Humidification • Hypothermia • Infection • Blockage of airway • Atelectasis Indications For Humidification • Provide humidity for dry gases • Correct humidity deficit in intubated or tracheostomized patients • Treatment of hypothermia • Correct bronchospasm induced by inspiration of cold air Factors Affecting Efficiency of Humidifiers • Temperature • Surface area of fluid exposed to water • Time of contact with water Basic Concepts • As gas travels through the lungs it achieves BTPS: – Body temp ~ 37C – Barometric pressure – Saturation with water vapor (100% relative humidity @ 37C) Basic Concepts • The point at which this occurs is called the isothermic saturation boundary (ISB) – Usually occurs ~ 5 cm below the carina – If the upper airway is bypassed or VE is significantly higher than norm, • The ISB will be deeper into the lungs and HUMIDITY therapy may be indicated Basic Physical Principles of Humidity • Humidity is essentially the water vapor in a gas. • This water vapor can be described in several ways, as: • 1. Absolute humidity - The actual content of water vapor in a gas measured in milligrams per liter. • 2. Potential humidity - The maximum amount of water vapor that a gas can hold at a given temperature. • 3. Relative humidity - The amount of water vapor in a gas as compared to the maximum amount possible, expressed as a percentage • 4. Body humidity - The absolute humidity in a volume of gas saturated at body temperature of 37 C; equivalent to 43.8 mg/L Formulas Used When Calculating Humidity • %RH=(absolute humidity/saturated capacity) x 100 • %BH = (absolute humidity/43.8mg/L) x 100 Primary Humidity Deficit • If the atmosphere's relative humidity is less than 100%, the air of the atmosphere has what is referred to as a humidity deficit. • If outside air at 20°C has 14 mg/l of water vapor, and needs to have 17.3 mg/l to be fully saturated, it is said to have a primary humidity deficit of 3.3 mg/l. – 17.3 mg/L (potential) – 14 mg/L (absolute) = 3.3 mg/L (primary deficit) • Remember that the potential is based temp • The primary humidity deficit occurs in the atmosphere and represents the difference between what humidity there is and what there could be. • Primary Humidity Deficit = Potential Water Vapor Content - Actual Water Vapor Content Secondary Humidity Deficit • This is the moisture deficit in the inspired air that the nose and upper airway need to compensate for. – The amount of water vapor the body needs to add to inspired air to achieve saturation at body temperature. • When air is breathed into the nasal cavity and heated to body temperature, its potential water vapor rises to 44 mg/l, which is the potential water vapor content of air at 37°C. • Therefore, unless the air of the atmosphere is at least 37°C and fully saturated, there exists a moisture deficit. • Secondary Humidity Deficit = 44 mg/l - Absolute Humidity. Water Losses • Insensible: skin and lungs • Sensible: urine, GI tract, sweat • Additive: vomiting, diarrhea, suction from intestines, severe burns, and fever • For each degree of temperature above 99F for over 24 hours, 1000m of fluid is required for replacement Water Vapor Correction • Water vapor acts in most ways like any other gas, it creates a partial pressure when it’s in a mixture of gases. • That partial pressure depends – The amount of water vapor present • Which in turn depends on the temperature. • Unlike other gases in the air, changes in the barometric pressure of the atmosphere under normal conditions do not have much impact on the partial pressure of water. Water Vapor Correction • As a result, it is best to calculate the partial pressures of the other gases in the air after the partial pressure of water vapor has been determined--especially when measuring the air within the lungs. • Inside the lungs, the partial pressure of water vapor is approximately 47 mm Hg. • This value is relatively constant because the air entering the lungs is normally saturated and at 37°C. • By subtracting the partial pressure of the water vapor from the total atmospheric pressure, you will find what is referred to as the dry gas pressure Importance of Humidity • It is needed to maintain normal bronchial hygiene • It promotes functions of the normal mucociliary escalator • It maintains the body's vital homeostasis • Without humidity: – the nearly 100 ml of mucus secreted daily would become quite thick and tenacious. – actual lung parenchyma would dry up, causing a loss of normal compliance which would restrict lung movement and reduce ventilation. Importance of Humidity If the upper airway were bypassed or dry gases were inhaled, a series of adverse reactions could occur, including: – Slowing of mucus movement – Inflammatory changes and possible necrosis of pulmonary epithelium – Retention of thick secretions and encrustation – Bacterial infiltration of mucosa (bronchitis) – Atelectasis – Pneumonia – Impairment of ciliary activity Importance of Humidity The general goals of humidity and aerosol therapy are to: 1. Promote bronchial hygiene 2. Loosen dried and/or thick secretions 3. Promote a effective coughs to clear secretions 4. Provide adequate humidity in the presence of an artificial airway 5. Deliver adequate humidity when administering dry gases therapies 6. Delivering prescribed medications Clinical Evaluation of the Need for Humidity and/or Aerosol Use • Patient's age and ability to move normal secretions • • • • Neuromuscular status Recent or planned surgeries Trauma Disease conditions • The presence of any of these may impair the patient's ability to cough and move secretions. Another problem may occur when patients develop very thick and abundant amounts of secretions which cannot be moved with normal muscle activity--making humidity or aerosol therapy necessary. • Indications for delivery of humidified gases and aerosols • Primary indications for humidifying inspired gases include: • Administration of medical gases • Delivery of gas to the bypassed upper airway • Thick secretions in nonintubated patients Indications for delivery of humidified gases and aerosols • Additional indications for warming inspired gases: – Hypothermia – Reactive airway response to cold inspired gas Sign/Symptoms of Inadequate Airway Humidification • • • • Atelectasis Dry, nonproductive cough Increased airway resistance Increased in incidence of infection • Increased work of breathing • Substernal pain • Thick, dehydrated secretions Humidification Devices • The purpose of humidifiers is to deliver a gas with a maximum amount of water vapor content. • May be heated or unheated, and the factors affecting the efficiency of humidification devices include: – temperature – time of exposure between gas and water – surface area involved in the gas/water contact Humidification Devices • As temperature rises, the force exerted by the water molecules increases, enabling their escape into the gas, adding to the humidity. – So the higher the tempthe more humidity • Longer exposure of a gas to the water increases the opportunity for the water molecules to evaporate during the humidifier's operation. • The greater the area of contact between water and gas, the more opportunity for evaporation to occur. Aerosol Therapy Basic Concepts Aerosol Therapy • It is important to remember that an aerosol is not the same as humidity. • Humidity is water in a gas in molecular form, while an aerosol is liquid or solid particles suspended in a gas. • Examples of aerosol particles can be seen everywhere: as pollen, spores, dust, smoke, smog, fog, mists, and viruses. Aerosol Therapy • Aerosol therapy is designed to increase the water content delivered while delivering drugs to the pulmonary tree • Deposition location is of vital concern • Some factors that affect aerosol deposition are aerosol particle size and particle number. Aerosol Output • The actual weight or mass of aerosol that is produced by nebulization. • Usually measured as mg/L/min also called aerosol density • Aerosol output does not predict aerosol delivery to desired site of action. Particle Size The particle size of an aerosol depends on the device used to generate it and the substance being aerosolized. Particles of this nature, between 0.005 and 50 microns, are considered an aerosol. The smaller the particle, the greater the chance it will be deposited in the tracheobronchial tree. Particles between 2 and 5 microns are optimal in size for depositing in the bronchi, trachea and pharynx. Particle Size • Heterodisperse: – aerosol with a wide range of particle sizes (medical aerosols) • Monodisperse: – aerosol consisting of particles similar in size (laboratory, industry) Deposition • The aerosol particles are retained in the mucosa of the respiratory tract. They get stuck! • The site of deposition depends on size, shape, motion and physical characteristics of the AIRWAYS Mechanism resulting in Deposition: Inertial Impaction • Moving particles collide with airway surface. – Large particles (>5micros), upper and large airways • Physics: the larger the particle, the more likely it will remain moving in a straight line even when the direction of flow changes. • Physics: greater velocity and turbulence results in greater tendency for deposition Mechanism resulting in Deposition Table: Particle size and area of deposition. Particle Size in Microns 1 to 0.25 1 to 2 2 to 5 5 to 100 Area of Deposition Minimal settling Enter alveoli with 95% deposition Deposit proximal to alveoli Trapped in nose and mouth Mechanism resulting in Deposition: Sedimentation • Particles settle out of aerosol suspension due to gravity. • The bigger it is the faster it settles! • Medium particles: 1-5 microns, central airways • Directly proportional to time. • The longer you hold your breath the greater the sedimentation Mechanism resulting in Deposition: Diffusion • Actual diffusion particles via the alveolar-capillary membrane and to a lesser extent tissue-capillary membranes of respiratory tract • Lower airways: 2-5 microns • Alveoli: 1-3 microns • These values are from your book Deposition of Particles is also affected by: • Gravity – – Gravity affects large particles more than small particles, causing them to rain-out. • Viscosity - The viscosity of the carrier gas plays an important role in deposition. • For example, if a gas like helium, which has a low viscosity and molecular weight, is used as a carrier gas, gravity will have more of an effect upon the aerosol. • Helium is very light and hence can't carry these particles well, leading to rain-out and early deposition. Deposition of Particles is also affected by: • Kinetic activity - As aerosolized particles become smaller, they begin to exhibit the properties of a gas, including the phenomenon of "Brownian movement." • This random movement of these small (below lmm) particles causes them to collide with each other and the surfaces of the surrounding structures, causing their deposition. • As particle size drops below 0.1m, they become more stable with less deposition and are exhaled. Deposition of Particles is also affected by: • Particle inertia (repeated) - Affects larger particles which are less likely to follow a course or pattern of flow that is not in a straight line. • As the tracheobronchial tree bifurcates, the course of gas flow is constantly changing, causing deposition of these large particles at the bifurcation. Deposition of Particles is also affected by: • Composition or nature of the aerosol particles Some particles absorb water, become large and rainout, while others evaporate, become smaller and are conducted further into the respiratory tree. • Hypertonic solutions absorb water from the respiratory tract, become larger and rain-out sooner. • Hypotonic solutions tends to lose water through evaporation and are carried deeper into the respiratory tract for deposition. • Isotonic solutions (0.9% NaCl) will remain fairly stable in size until they are deposited. Deposition of Particles is also affected by: • Heating and humidifying - As aerosols enter a warm humidified gas stream, the particle size of these aerosols will increase due to the cooling of the gas in transit to the patient. • This occurs because of the warm humidified gas cooling and depositing liquid (humidity) upon the aerosol particles through condensation. Deposition of Particles is also affected by: • Ventilatory pattern - RCPs easily control this by simple observation and instruction. • For maximum deposition, the patient must be instructed to: – Take a slow, deep breath. – Inhale through an open mouth (not through the nose). – At the end of inspiration, use an inspiratory pause, if possible, to provide maximum deposition. – Follow with a slow, complete exhalation through the mouth. Aerosol vs. Systemic • In many cases, aerosols are superior in terms of efficacy and safety to the same systemically administered drugs used to treat pulmonary disorders. • Aerosols deliver a high concentration of the drugs with a minimum of systemic side effects. • As a result, aerosol drug delivery has a high therapeutic index; especially since they can be delivered using small, large volume, and metered dose nebulizers. Aerosol delivery is accomplished in a variety of ways: nasal spray pump metered-dose inhaler (MDI) dry powder inhaler (DPI) jet nebulizer small volume nebulizer (SVN) large volume nebulizer small-particle aerosol generator (SPAG) mainstream nebulizers ultrasonic nebulizer (USN) intermittent positive pressure breathing (IPPB) devices Formula Review • PAO2 (page 55) • FIO2(PB-PH2O) – PaCO2/0.8 • This formula represents the amount of partial pressure of Oxygen that is in a patients lung • Normal value is approximately 100 given normal conditions FIO2 is the inspired fractional oxygen concentration. When breathing room air this is 21% Formula Review • PAO2 (page 55) • FIO2(PB-PH2O) – PaCO2/0.8 • PB is the atmospheric pressure, at sea level this is 760 mmhg • PH2O is the water vapor pressure in the lung. Under normal conditions this is 47 mmHg • PaCO2 is the partial pressure of CO2 in a patients arterial blood. Obtained through a blood draw from the artery • 0.8 is a factor which represents the amount of O2 vs Co2 is produced by the body Formula Review • PAO2 (page 55) • FIO2(PB-PH2O) – PaCO2/0.8 Normal values will vary as a patient is: • On supplemental FIO2 greater than 21% • In environment where the atmospheric pressure is higher or lower • In a state where they are inhaling dry air • Have an increase in their PaCo2 Formula Review • http://www.youtube.com/watch?v=zZX9jJqSl Qs • http://www.youtube.com/watch?v=xH5Y3Km x82w • http://www.youtube.com/watch?v=nRpwdw m06Ic Formula Review • CaO2 (Total Oxygen Content) • This formula is used to determine the amount of oxygen that is available to the tissues • CaO2 = (Hb x 1.34 x SaO2) + (PaO2 x 0.003) • Hb= Hemoglobin (carries O2) • 1.34 (sometimes 1.36 is used) carrying capacity of Hb for O2 is 1.34ml O2 / Gram Hb • SaO2= Saturation of Hb with Oxygen, obtained from a ABG. HBO2/Total Hb Formula Review • CaO2 (Total Oxygen Content) • (Hb x 1.34 x SaO2) = the amount of O2 binded with Hb • (PaO2 x 0.003)= amount of O2 dissolved in plasma • PaO2 = partial pressure of Oxygen found in arterial blood, obtained with a ABG • Normal values: (14 x 1.34 x .97) + (90 x 0.003) O2 Content • http://www.youtube.com/watch?v=BQCNrVZ BobU Formula Review • DO2 • The delivery of oxygen to the tissues per minute is calculated from: DO2 = [1.39 x Hb x SaO2 + (0.003 x PaO2)] x Q • Q = Cardiac Output Formula Review • The amount of oxygen in the blood: the oxygen binding capacity of hemoglobin x the concentration of hemoglobin x the saturation of hemoglobin + the amount of dissolved oxygen all Multiplied by the Cardiac Output (Q). • The cardiac output is determined by preload, afterload and contractility. • The hemoglobin concentration is determined by production, destruction and loss. Formula Review • CvO2 • The amount of O2 in the venous blood (left over after cellular metabolism) • CvO2 used to estimate Oxygen consumption when compared to CaO2 • CvO2 = (Hb x 1.34 x SVO2) + (PvO2 x 0.003) • Oxygen Consumption (VO2)= C(a-v)QT • *QT= Cardiac Output