Notes- Relative Humidity
advertisement
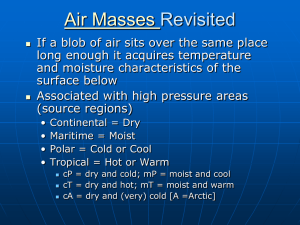
Question of the Day Question: What causes the greenhouse effect and why is it important? Answer: … … … p. 605 CYU 2-7 p. 605 CYU 2-7 2. Identify which of the following gases is most abundant in Earth’s atmosphere today. a. argon c. oxygen b. nitrogen d. carbon dioxide 2. Nitrogen is most abundant in Earth’s atmosphere today. 3. Compare Earth’s early atmosphere with its present atmosphere. 3. Earth’s early atmosphere was composed of many gases that would be poisonous to us today. As life forms evolved and began to photosynthesize, oxygen was produced. Once animals adapted to breathing oxygen, they began to give off carbon dioxide, balancing the production of oxygen. p. 605 CYU 2-7 4. Arrange the steps of the oxygen-carbon dioxide cycle in the correct order: a. b. c. d. Animals breathe oxygen Plants produce oxygen Plants use carbon dioxide Animals exhale carbon dioxide 4. The correct order is b, a, d, c. b. a. d. c. Plants produce oxygen Animals breathe oxygen Animals exhale carbon dioxide Plants use carbon dioxide p. 605 CYU 2-7 5. Explain why the following statement is incorrect: global warming could cause oceans to rise, so the greenhouse effect must be eliminated completely. 5. If we were to eliminate the greenhouse effect completely, the world’s climate would become too cold for humans to survive. p. 605 CYU 2-7 6. Predict how much colder it is at the top of Mount Everest, which is almost 9km above sea level, than it is at the Indian coastline. Consider only the difference in altitude. (Hint: the temperature in the troposphere decreases by 6°C/km) 6. The temperature at the top of Mount Everest is about 54°C colder. 9km x 6°C/km = 54°C p. 605 CYU 2-7 7. In 1982, Larry Walters rose to an altitude of approximately 4900m (just over 3 miles) on a lawn chair attached to 45 helium-filled weather balloons. Give two reasons why Walters’s efforts were dangerous. 7. Walters’s efforts were dangerous because the oxygen content of the air is considerably lower at that altitude and the temperature is extremely cold. Relative Humidity Humidity the measure of the amount of water (moisture) in the air high humidity = lots of water in the air Saturated when the air is holding the maximum amount of moisture that it can hold at a particular temperature warm air can hold more water vapor than cold air Absolute Humidity actual amount of water in a given volume of air Units are g/mL Capacity The maximum amount of water the air can hold at a specific temperature If the air is holding its full capacity of water vapor it is saturated. Relative Humidity how much water is in the air relative (compared) to its capacity at a specific temperature = Absolute Humidity X 100 Capacity at a Particular Temperature Psychrometer/Hygrometer instrument used to measure relative humidity contains both a wet and a dry bulb thermometer Dew Point Temperature to which the air would have to be cooled to make the relative humidity 100% condensation can begin at this temperature Dew Point if below freezing, water will go from vapor to ice crystal the higher the temperature the more moisture it takes to reach the dew point In general: – High dew points = high humidity – Low dew points = low humidity