US 505(b)(2) Regulatory
Pathway and Strategies
Industry Perspectives
AAPS Regulatory Sciences Open Forum
11/16/2010
Ruth Stevens, PhD, MBA
Tale of Two Cliffs
• Big Pharma Patents
Lipitor
Flomax
Plavix
100 others
2
Tale of Two Cliffs
• Big Pharma Patents
• Generics
Small
molecules
3
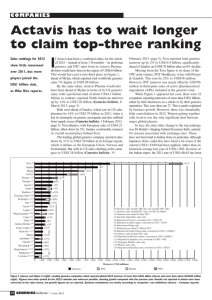
Differentiated Products Now Essentially Drive
Economics of US Generics: Mylan
Percent incremental contribution to 2008 cash EPS (%)
10
4
2
2
1
9
16
Differentiated
products
contributed**
~92% of ‘08
cash EPS
100
27
*Base EPS includes commodity generics, O-US generics, Matrix
**Incremental contribution
Reference: Ronny Gal, Research Analyst, Sanford C. Bernstein & Co. Presentation at
4
GPhA 2009 Annual Meeting
Total EPS (%)
Base* EPS
Sular
Bystolic
Royalties
Levothyroxine
Plendil
Ditropan XL
Paxil CR
EpiPen
Duragesic
29
505(b)(2) Process
Potential Regulatory Pathways for Drug Products
Under Development
Appropriate for drug
products that are the same
as approved products
Hybrid between an ANDA
[505(j)] and a full NDA
[505(b)(1)]
‘Full’ Application – Data
predominantly obtained from
studies conducted by the
Sponsor
505(j)
ANDA
505(b)(2) NDA
505(b)(1) NDA
5
505(b)(2) Regulation
Potential 505(b)(2) Candidates:
•
•
•
•
•
•
•
•
•
Marketed, unapproved (DESI) drug*
PET drugs (FDG F 18, Ammonia N 13, Sodium Fluoride F 18)
Racemates
Known excipients used as active drugs*
New dosage form
Pharmacokinetic alteration
New indication
New combination
Pro-drug of approved drug
* May or may not have an RLD
6
Defining the 505(b)(2) Application
A 505(b)(2) application is one for which one or more of the investigations
relied upon by the applicant for approval "were not conducted by or for
the applicant and for which the applicant has not obtained a right of
reference or use from the person by or for whom the investigations
were conducted" (21 U.S.C. 355(b)(2)).
7
505(b)(2) NDA
Reference Listed Drug (RLD)
• A drug product that has been previously
approved by the FDA
• Listed in the Orange Book
• Approval documents contain some of the
information needed for your 505(b)(2)
8
Approved Product Labeling
Drug Substance
Indication
Safety
Clinical
Pharmacology
DDI
Pharmacokinetics
Reproductive
9
Industry Examples
Highlighting the Benefits of utilizing the
505(b)(2) Regulatory Pathway
10
Corporate Strategy
The company applies its KME™ development model to identify existing drugs or
‘known molecular entities’ with established safety profiles which can be developed
and clinically differentiated for gastrointestinal indications. The KME™ development
model allows the company to more quickly establish the efficacy profile of its
candidates for the target gastrointestinal indications.
• 505(b)(2) provides the ability to clinically differentiate from other products
• Allows for market niche
• Not available for ANDA (505(j)) products
11
11
Corporate Strategy
Mr. Sims: Actually, because we start with drugs already approved by the FDA, we can
file for approval using the 505b2 procedure rather than the full NDA (new drug
application). Where clinical trials are required for a 505b2 application, the number of
patients required and length of time needed for those clinical trials are significantly less
than for a new chemical entity. For example, with ESTRASORB, our Phase 1 trial had
14 patients and our Phase 2 and Phase 3 trials required less than 400 patients. As the
efficacy and safety of the active ingredients have already been established, the
complexity of the studies, including the duration, is generally significantly less than for
an NDA application. We believe that from the starting point of developing a drug in a
micellar nanoparticle to regulatory submission, it should take no more than a three or
four year time frame, which is actually quite fast for this industry. From the point of
filing, we would add about another year for FDA review and approval.
• Eliminate some of the studies required for a 505(b)(1)
(preclinical, extensive safety and efficacy studies)
• Significant financial savings
• Significant time savings
http://www.novavax.com/images/TWST%200205%20Final.pdf
12
©2005 The Wall Street Transcript, 67 Wall Street, NYC 10005
Tel: (212) 952-7400 • Fax: (212) 668-9842 • Website: www.twst.com
Case Study: ColcrysTM - DESI
• Colchicine Tablets for treatment of acute gout and familial
Mediterranean fever (Mutual Pharmaceuticals, NDAs 22-351 and
22-352, July 2009)
• Colchicine injection removed from the market in February, 2008
because of often-fatal toxicity
– Mutual showed that this toxicity was due to excessive dose
and/or concomitant medications
• Approved based only on literature for efficacy for the treatment of
familial Mediterranean fever (FMF) and a single Phase 3 study for
the treatment of acute gout
13
Case Study: UlesfiaTM - Excipient
•
5% benzyl alcohol lotion for treatment of head lice (Sciele Pharma, NDA
22-129, April 9, 2009)
• Widely-used chemical in cosmetic industry
– Never approved as active ingredient so FDA classified it as NME
•
Development program:
– Nonclinical
• Literature for repeat dose and genetic toxicology
• Literature for in vitro studies demonstrating mechanism of action
• 2-year toxicology and carcinogenesis studies from the National Toxicology Program
– Clinical Pharmacology/Biopharmaceutics
• 2 PK studies (2nd was done because presence of API in the catheter wash was
confounded results of the 1st study)
– Efficacy
• 2 Phase 3 trials, 615 subjects total (240 on treatment)
– Safety
• Relied on its database of 8 studies (2 Phase 3, 3 Phase 2, 2 Phase 1, 1 special safety
study)
• Extensive publication review
Received 5 years data exclusivity
14
Case Study: Cafcit
• Caffeine citrate for treatment of apnea of prematurity (Mead
Johnson, NDA 20-793, September 21, 1999)
• Development Program:
– Submitted 1 double-blind trial in 58 preterm infants and 90 literature
articles
• Human PK studies from literature (19 articles)
• Drug-drug interactions from literature (71 articles)
– No human PK studies conducted in premature infants
• Used plasma caffeine levels from subjects in the study and used special software to do
population PK analysis
Received orphan drug status (7 years data exclusivity) and expedited review
15
Pro-Drugs
• Fundamental:
– Where does the pro-drug become the RLD?
–
16
Case Study: Valacyclovir
• Oral tablet for treatment of herpes
–
–
–
–
Pro-drug of acyclovir
Pro-drug in systemic circulation for about 30 min
Hepatic esterases convert pro-drug to acyclovir
Similar plasma metabolic profile to acyclovir after
conversion
17
Valacyclovir Regulatory Path
• 505(b)(2) NDA as a new molecular entity
– Acyclovir is RLD
• Can rely on some acyclovir nonclinical & clinical data
–
–
–
–
Nonclinical studies for NME
Full Phase 1 PK program
Phase 2 dose-ranging
1 adequate and well-controlled Phase 3 study
• 5 Years marketing exclusivity
18
505(b)(2) Benefits
• Able to earn exclusivity
– 3 years: clinical studies
essential for approval
– 5 years: NCE – old drugs
never approved under an NDA
– 7 years: Orphan Drug
19
Benefits of 505(b)(2)
• Get out of competitive environment of Generics
• Regulatory Review Period (10 months)
• May be attractive for investors
– 505(b)(2) allows for clinical differentiation
– Market potential may be greater than generics
20
505(b)(2) Risks
• Imprecise development costs and timelines
– ANDA’s have few re-do’s of BE
– Recruitment of patients not naive
– Uncertain dose (Phase 2)
• Unknown competition
– Other companies can target the same opportunity
21
505(b)(2) Risks
• Uncertain market acceptance
–
–
–
–
Formulary
Sales Force
Doctors
Patients
22
505(b)(2) Risks
• May not be attractive for investors
– “Generics have less risks and better returns”
• VC’s don’t understand 505(b)(2)
• ROI may be lower than 505(b)(1), or a total payoff may be
lower (double $50MM or $500MM)
23
505(b)(2) Risks
• Like Generics (505(j)):
– must include patent certification(s)
– include all relevant patent(s)
– subject to the same Paragraph IV challenge and
litigation including a 30-month stay, but it is not
granted a 180-day exclusivity
24
Decision to Pursue the 505(b)(2) Regulatory Pathway
Considerations:
•
•
•
•
Willingness to take educated risks
In-house Expertise
Company Culture
Historical Precedence
25
Thank You
• Regulatory Sciences Co-Moderators
– Michael Bornstein, PhD
– Annette Bak, PhD, MBA
• Rajneesh Taneja, PhD, RPh
26