Stroke - Anticoagulation Centers of Excellence

PREVENTING
Atrial Fibrillation Related
STROKES
with Anticoagulants
September 2012 - June 2013
Disclosure of Commercial Support
PREVENTING
Atrial Fibrillation Related
STROKES with Anticoagulants
This activity is supported by educational grants from
Boehringer Ingelheim Pharmaceuticals, Inc. and Bristol-Myers
Squibb and Pfizer Inc.
This slide presentation and artwork was independently developed by Boston University School of Medicine’s
Powerpoint designer.
Boston University School of Medicine’s Disclosure Policy
Boston University School of Medicine asks all individuals involved in the development and presentation of Continuing Medical Education (CME) activities to disclose all relationships with commercial interests. This information is disclosed to CME activity participants. Boston University School of Medicine has procedures to resolve any apparent conflicts of interest. In addition, faculty members are asked to disclose when any unapproved use of pharmaceuticals and devices is being discussed.
2
Accreditation Information
PREVENTING
Atrial Fibrillation Related
STROKES with Anticoagulants
This activity has been planned and implemented in accordance with the Essential Areas and Policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint sponsorship of Boston University School of Medicine and
Anticoagulation Forum. Boston University School of Medicine is accredited by the ACCME to provide continuing medical education for physicians.
Boston University School of Medicine designates this live activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™.
Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Continuing Nursing Education Provider Unit, Boston University School of Medicine is accredited as a provider of continuing nursing education by the American Nurses Credentialing Center’s Commission on Accreditation.
CNE Contact Hours: 1.00
Nurses will receive contact hours for those sessions attended, after completion of an evaluation and claim for credit form.
Continuing Pharmacy Education Credits
The University of Rhode Island College of Pharmacy is accredited by the Accreditation Council for
Pharmacy Education as a provider of continuing pharmacy education. Attendance and completion of program evaluations at the conclusion of the program are required for a statement of credit. This knowledge-based activity is approved for 1.0 Contact Hours (0.1 CEUs). UAN: 0060-9999-12- 040-L01-P.
Expiration date: September 5, 2013.
3
Learning Objectives
PREVENTING
Atrial Fibrillation Related
STROKES with Anticoagulants
At the conclusion of this activity participants will be able to:
:
• Describe benefits of oral anticoagulants for stroke prevention in atrial fibrillation
• Identify the population of patients who would be at risk of stroke with atrial fibrillation
• Compare current and new oral anticoagulants with regards to safety, efficacy, pharmacology, cost and convenience
• Compare the benefits and risks of oral anticoagulant therapy for reducing the risk of stroke in atrial fibrillation patients
• Utilize available decision making tools to stratify the risks and benefits of anticoagulation therapy in patients with atrial fibrillation
4
Highlights
PREVENTING
Atrial Fibrillation Related
STROKES with Anticoagulants
• Prevalence and incidence of AF
• Risk stratification for stroke and bleeding
• New oral anticoagulants
• Guidelines
• Practical considerations for choosing an anticoagulant
Highlights
PREVENTING
Atrial Fibrillation Related
STROKES with Anticoagulants
• Prevalence and incidence of AF
• Risk stratification for stroke and bleeding
• New oral anticoagulants
• Guidelines
• Practical considerations for choosing an anticoagulant
Question #1
An 82 year old man is in your office for an annual
Medicare physical. What is the chance he has atrial fibrillation?
1.
2.
3.
4.
1%
5%
10%
25%
7
Prevalence of Diagnosed AF
Stratified by Age and Sex
12.0
10.0
Women
Men 10.3
9.1
11.1
8.0
7.3
7.2
6.0
5.0
5.0
4.0
3.4
3.0
2.0
1.0
1.7
1.7
0.1
0.2
0.4
0.9
0.0
<55 55-59 60-64 65-69 70-74 75-79 80-84
# Women
# Men
530
1529
310
634
566
934
896
1426
1498
1907
Go AS, JAMA. 2001 May 9;285(18):2370-5. Pub Med PMID: 11343485
1572
1886
1291
1374
> 85
1132
759 x-axis = % y-axis = # of men/women
8
Question #2
A 46 year old male patient is in for an annual physical exam. What is his lifetime risk of developing AF?
1.
2.
1%
5%
3.
4.
10%
25%
9
Incidence of AF
Lifetime Risk for AF at Selected Index Ages by Sex
Index Age, yrs
40
50
60
70
80
Men
26.0% (24.0 – 27.0)
25.9% (23.9 – 27.0)
25.8% (23.7 – 26.9)
24.3% (22.1 – 25.5)
22.7% (20.1 – 24.1)
Women
23.0% (21.0 – 24.0)
23.2% (21.3 – 24.3)
23.4% (21.4 – 24.4)
23.0% (20.9 – 24.1)
21.6% (19.3 – 22.7)
1 in
4
Men & women
>40 Years will develop AF
Lifetime risk if currently free of AF
Lloyd-Jones DM, et al. Circulation. 2004 Aug 31;110(9):1042-6. Pub Med PMID: 15313941.
10
Highlights
PREVENTING
Atrial Fibrillation Related
STROKES with Anticoagulants
• Prevalence and incidence of AF
• Risk stratification for stroke and bleeding
• New oral anticoagulants
• Guidelines
• Practical considerations for choosing an anticoagulant
Question #3
68 year old female with atrial fibrillation and no other comorbidities. How would you classify her stroke risk?
1.
2.
3.
Low
Moderate
High
12
Scoring Systems in Atrial Fibrillation
• Given that anticoagulant therapy has both risks
(principally bleeding) and benefits (a reduced risk of thrombosis) many authors have attempted to produce scoring systems which estimate the risks of these outcomes
• No one scoring system is universally accepted or highly predictive (in individual patients)
13
Scoring Systems in Stroke Risk
• A variety of systems have been published
– Outlined on next slide
• All use selected clinical characteristics to predict the risk of stroke
• Most widely used is the CHADS
2 score
• All scores provide a rough estimate of risk of thrombosis in a population at similar risk as patient being reviewed
14
Atrial Fibrillation Risk Stratification
12 Schemes applied to 1000 patients from SPAF III study
High Moderate Low
Stroke Risk in Atrial Fibrillation Working Group. Stroke. 2008 Jun;39(6):1901-10. Pub Med PMID: 18420954. 15
CHADS
2
: Risk of Stroke
National Registry of Atrial Fibrillation Participants (NRAF)
CHADS
2
Score
0
# Patients
(n = 1733)
120
# Strokes
(n = 94)
2
NRAF Crude
Stroke Rate per
100 Patient-yrs
1.2
NRAF Adjusted
Stroke Rate
(95% CI)†
1.9 (1.2-3.0)
1
2
3
4
463
523
337
220
17
23
25
19
2.8
3.6
6.4
8.0
2.8
4.0
5.9
8.5
(2.0-3.8)
(3.1-5.1)
(4.6-7.3)
(6.3-11.1)
5 65 6 7.7
12.5 (8.2-17.5)
6 5 2 44.0
18.2 (10.5-27.4)
Scoring:
1 point: Congestive heart failure, HTN, < 75 years, and DM
2 points: Stroke history or transient ischemic attack
† Expected stroke rate per 100 pt-yrs from the exponential survival model, assuming aspirin not taken
Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. JAMA. 2001 Jun 13;285(22):2864-70.
Pub Med PMID: 11401607.
16
CHA
2
DS
2
-VASc
2009 Birmingham Schema Expressed as a Point-Based Scoring System
Risk Factor
C ongestive heart failure/LV dysfunction
H ypertension
A ge ≥ 75 y
D iabetes mellitus
S troke/TIA/TE
V ascular disease
(prior myocardial infarction, peripheral artery disease, or aortic plaque)
A ge 65-74 y
S ex c ategory
(i.e. female gender)
LV = left ventricular; TE = thromboembolism
Score
2
1
1
1
2
1
1
1
Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Chest. 2010 Feb;137(2):263-72. Pub Med PMID: 19762550. 17
CHA
2
DS
2
-VASc
Stroke or Other TE at One Year
CHA
2
DS
VASc
2
Score
-
0
1
4
5
2
3
6
7
#
103
162
184
203
208
95
57
25
#TE
Events
0
1
4
3
3
8
2
2
TE Rate During
1 yr (95% CI)
0% (0-0)
0.6% (0.0-3.4)
1.6% (0.3-4.7)
3.9% (1.7-7.6)
1.9% (0.5-4.9)
3.2% (0.7-9.0)
3.6% (0.4-12.3)
8.0% (1.0-26.0)
TE Rate During 1 yr,
Adjusted for
Aspirin RX
0%
0.7%
1.9%
4.7%
2.3%
3.9%
4.5%
10.1%
8
9
9
1
1
1
11.1%
100%
(0.3-48.3)
(2.5-100)
14.2%
100%
Total 1,084 25 P Value for trend 0.003
Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Chest. 2010 Feb;137(2):263-72. Pub Med PMID: 19762550. 18
CHA
2
DS
2
-VASc and CHADS
2
Score 0
Refines stroke risk stratification in AF patients: nationwide cohort
–1
1 Year Follow-up 12 Years Follow-up
Person Yrs Events Stroke rate (95%CI) Person Yrs Events Stroke rate (95%CI)
CHADS
2 score 0 –1 40,272 1,405 3.49 (3.31
–3.68) 187,200 4,599 2.46 (2.39
–2.53)
CHA
2
DS
2
-VASc = 0 6,919 58 0.84 (0.65
–1.08) 39,500 299 0.76 (0.68
–0.85)
CHA
2
DS
2
-VASc = 1 8,880 159 1.79 (1.53
–2.09) 45,926 662 1.44 (1.34
–1.56)
CHA
2
DS
2
-VASc = 2 11,863 435 3.67 (3.34
–4.03) 51,595 1,489 2.89 (2.74
–3.04)
CHA
2
DS
2
-VASc = 3 11,473 660 5.75 (5.33
–6.21) 45,799 1,933 4.22 (4.04
–4.41)
CHA
2
DS
2
-VASc = 4 1,137 93 8.18 (6.68
–10.02) 4,380 216 4.93 (4.32
–5.64)
CHADS
2 score = 0 17,327 275 1.59 (1.41
–1.79) 92,531 1182 1.28 (1.21
–1.35)
CHA
2
DS
2
-VASc = 0 6,919 58 0.84 (0.65
–1.08) 39,500 299 0.76 (0.68
–0.85)
CHA
2
DS
2
-VASc = 1 6,811 119 1.75 (1.46
–2.09) 35,079 504 1.44 (1.32
–1.57)
CHA
2
DS
2
-VASc = 2 3,347 90 2.69 (2.19
–3.31) 16,710 353 2.11 (1.90
–2.34)
CHA
2
DS
2
-VASc = 3 250 8 3.20 (1.60
–6.40) 1,242 26 2.09 (1.43
–3.07)
CHADS
2
Score = 1 22,945 1,130 4.92 (4.65
–5.22) 94,669 3417 3.61 (3.49
–3.73)
CHA
2
DS
2
-VASc = 1 2,069 40 1.93 (1.42
–2.64) 10,847 158 1.46 (1.25
–1.70)
CHA
2
DS
2
-VASc = 2 8,516 345 4.05 (3.65
–4.50) 34,885 1136 3.26 (3.07
–3.45)
CHA
2
DS
2
-VASc = 3 11,223 652 5.81 (5.38
–6.27) 44,557 1907 4.28 (4.09
–4.48)
CHA
2
DS
2
-VASc = 4 1,137 93 8.18 (6.68
–10.02) 4,380 216 4.93 (4.32
–5.64)
Olesen JB, Torp-Pedersen C, Hansen ML, Lip GY. Thromb Haemost. 2012 Jun;107(6):1172-9. Pub Med PMID: 22473219.
19
Question #4
78 year old male with atrial fibrillation and hypertension
(CHADS2 score = 2 [4% stroke rate per year]). What is his annual major bleeding rate?
1.
1%
2.
3.
2%
3%
4.
5.
5%
10%
20
Bleeding Risk Scores
• Variety of scoring systems developed to predict risk of bleeding in patients initiating anticoagulants, as with stroke risk
• Less predictive than stroke risk scores, in general
• Each score incorporates clinical characteristics and provides estimate of risk of bleeding in a population similar to patients being considered
• Unclear whether to include risk scores in decision making for individual patients
21
Bleeding Risk Scores Widely Used in AF
•
HAEMORRHAGES
1
•
HASBLED
2
•
ATRIA Score
3
1.
Gage BF, et al. Am Heart J. 2006 Mar;151(3):713-9. PMID: 16504638. Pub Med PMID:16504638.
2.
Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. Chest. 2010 Nov;138(5):1093-100. PMID:20299623.
3.
Fang MC, et al. J Am Coll Cardiol. 2011 Jul 19;58(4):395-401. Pub Med PMID:21757117.
22
Bleeding Risk Scores in AF
ATRIA
Anemia 1 3
HAS-BLED
H ypertension 4 1
HEMORR
2
HAGES
H epatic 10 or
Renal disease 2
Severe renal disease 2
Age ≥75 yrs
Any prior hemorrhage
Hypertension 3
3
2
1
1
A bnormal Renal 5 or
Liver function 6
S
B
L troke leeding abile INR 8
1
1
1
1
1
E thanol abuse
M alignancy
O lder Age (>75 yrs)
R educed platelet number or function 11
E lderly (>65 yrs) 1 R ebleeding 12
1.
Hemoglobin <13 g/dl men; <12 g/dl women
2.
Estimated glomerular filtration rate <30 ml/min or dialysis-dependent
D rugs 9
Alcohol or 1
1
3.
Diagnosed hypertension
4.
Systolic blood pressure >160 mmHg
5.
Presence of chronic dialysis or renal transplantation or serum creatinine ≥200 mmol/L
6.
Chronic hepatic disease (eg cirrhosis) or biochemical evidence of significant hepatic derangement (eg bilirubin 2 x upper limit of normal, in association with aspartate aminotransferase/alanine aminotransferase/alkaline phosphatase >3 x upper limit normal, etc.)
8.
Unstable/high INRs or poor time in therapeutic range (eg <60%)
9.
Concomitant use of drugs, such as antiplatelet agents, non-steroidal anti-inflammatory drugs, or alcohol abuse etc.
10. Cirrhosis, two-fold or greater elevation of AST or APT, or albumin <3.6 g/dl
11. Platelets <75,000, use of antiplatelet therapy (eg daily aspirin) or NSAID therapy; or blood dyscrasia
12. Prior hospitalization for bleeding
13. Most recent hematocrit <30 or hemoglobin <10 g/dl
14. CYP2C9*2 and/or CYP2C9*3
15. Alzheimer's dementia, Parkinson's disease, schizophrenia, or any condition predisposing to repeated falls
H
A
G
E
S ypertension nemia troke
13
4 enetic factors 14 xcessive fall risk 15
Apostolakis S, Lane DA, Guo Y, Buller H, Lip GY. J Am Coll Cardiol 2012;60:000 –000. 2012 Jul 24. [Epub ahead of print]
Online Appendix. PMID: 22858389.
1
1
1
1
2
1
1
1
1
1
1
1
23
AMADEUS Cohort
Stratified by the HEMORR
2
HAGES, HAS-BLED, and ATRIA Schemes
All Patients
Clinically
Relevant
Bleeding
Major
Bleeding Scheme
HEMORR
2
HAGES
Low (≤1) Risk
Intermediate Risk (2
–3)
High Risk (>3)
TOTAL
HAS-BLED
Low Risk (<3)
High Risk (
≥3)
TOTAL
ATRIA
Low Risk (<4)
Intermediate Risk (4)
High Risk (>4)
TOTAL
1,738 (76.6)
517 (22.8)
13 (0.5)
2,268
1,739 (75.9)
553 (24.1)
2,292
2,038 (90)
102 (4.4)
128 (5.6)
2,268
182 (10.5)
63 (12.2)
3 (23.1)
248 (10.9)
159 (9.1)
92 (16.6)
251 (11.0)
220 (10.8)
13 (12.7)
18 (14.1)
248 (10.9)
25 (1.4)
13 (2.5)
1 (7.7)
39 (1.7)
22 (1.3)
17 (3.1)
39 (1.7)
31 (1.5)
3 (2.9)
5 (3.9)
39 (1.7)
Apostolakis S, Lane DA, Guo Y, Buller H, Lip GY. J Am Coll Cardiol 2012;60:000 –000. 2012 Jul 24. [Epub ahead of print]
Online Appendix. PMID: 22858389.
24
Risks of Bleeding with Warfarin or
Dabigatran in AF
Oldgren J, et al. Ann Intern Med. 2011 Nov 15;155(10):660-7, W204. Pub Med PMID: 22084332.
25
Adjusted HR for Death After Stroke,
MI, or Major Hemorrhage
In Patients Who Received Antiplatelet Therapy in the ACTIVE Trials
Event Pts With
Event, n
Subsequent
Deaths , n
(Adjusted Rate)
HR for Death
(95% CI)†
Relative
Weights‡
Ischemic stroke
Hemorrhage stroke
Subdural hemorrhage
Major extracranial bleeding event
Myocardial infarction
† Compared to no event
‡ ratio of hazard ratios
785
59
42
435
260
362 (36.4)
48 (81.4)
15 (32.4)
162 (31.6)
120 (38.9)
5.74
(5.10
– 6.47)
1.00
(reference)
17.67
(13.15
– 23.75)
3.44
(2.06
– 5.74)
3.08
0.60
3.82
(3.24 – 4.51)
5.44
(4.51
– 6.56)
0.67
0.95
Connolly SJ, et al. Ann Intern Med. 2011 Nov 1;155(9):579-86. Pub Med PMID: 22041946.
26
Highlights
PREVENTING
Atrial Fibrillation Related
STROKES with Anticoagulants
• Prevalence and incidence of AF
• Risk stratification for stroke and bleeding
• New oral anticoagulants
• Guidelines
• Practical considerations for choosing an anticoagulant
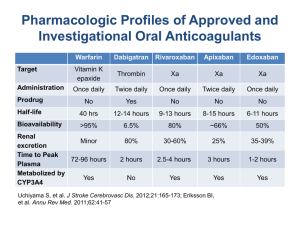
Pharmacokinetics of NOACs
Direct factor inhibition
Bioavailability (F rel
)
Peak action (t max
)
Protein binding
Renal clearance
Elimination half life with creatinine clearance > 80 ml/min
Elimination half life with creatinine clearance 50 –79 ml/min
Elimination half life with creatinine clearance 30
–49 ml/min
Elimination half life with creatinine clearance < 30 ml/min
Apixaban Dabigatran Rivaroxaban
Xa
80%
1 –3 hr
84%
25%
IIa
6%
1 –3 hr
35%
80%
Xa
80%
1 –3 hr
92
–95%
33%
15.1 hr
14.6 hr
17.6 hr
17.3 hr
13.8 hr
16.6 hr
18.7 hr
27.5 hr
8.3 hr
8.7 hr
9.0 hr
9.5 hr
Kaatz S, et al. Am J Hematol. 2012 May;87 Suppl 1:S141-5. Pub Med PMID: 22473649.
28
Measuring the Effect of NOACs
Coagulation Assays Apixaban Rivaroxaban Dabigatran
PT
-dilute PT
-modified PT
Not useful
Data n/a
Qualitative
Qualitative
Data n/a
Data n/a
Not useful
Data n/a
Data n/a aPTT
TT
-dTT/HEMOCLOT
Chromogenic Assays
-Anti-Xa
-Anti-Iia n/a = not available
Not useful
No effect
No effect
Quantitative
No effect
Not useful
No effect
No effect
Quantitative
No Effect
Qualitative
Qualitative
Quantitative
No effect
Quantitative
Garcia DA, et al. In review.
29
Reversal of NOACs
Types of Studies Evaluating Reversal of New Oral Anticoagulants
Oral activated charcoal
Hemodialysis
Hemoperfusion with activated charcoal
Fresh frozen plasma
Activated factor VIIa
3-factor PCC
4-factor PCC
Apixaban Dabigatran
No data
No data
No data
No data
No data
No data
No data
Rivaroxaban
In vitro
Human volunteers
In vitro
Mouse model
Rat model
No data
Human volunteers and rat model
No data
No data
No data
No data
Rat and baboon model
No data
Human volunteers
Kaatz S, et al. Am J Hematol. 2012 May;87 Suppl 1:S141-5. Pub Med PMID: 22473649.
30
Reversal of NOACs
Suggestions for Reversal of New Oral Anticoagulants
Oral activated charcoal
Hemodialysis
Hemoperfusion with activated charcoal
Fresh frozen plasma
Activated factor VIIa
3-factor PCC
4-factor PCC
Apixaban Dabigatran
Yes
No
Possible
No
Unclear
Unclear
Possible
Yes
Yes
Yes
No
Unclear
Unclear
Possible
Rivaroxaban
Yes
No
Possible
No
Unclear
Unclear
Possible
Kaatz S, et al. Am J Hematol. 2012 May;87 Suppl 1:S141-5. Pub Med PMID: 22473649.
31
Meta-analysis of Efficacy and Safety of
New Oral Anticoagulants
Dabigatran, Rivaroxaban, Apixaban vs. Warfarin in AF patients
All cause stroke/SEE
Ischemic and unspecified stroke
Hemorrhagic stroke
Meta-analysis of Efficacy and Safety of
New Oral Anticoagulants
Dabigatran, Rivaroxaban, Apixaban vs. Warfarin in AF patients
Major bleeding
Intracranial bleeding
GI Bleeding
Miller CS, Grandi SM, Shimony A, Filion KB, Eisenberg MJ. Am J Cardiol. 2012 Aug 1;110(3):453-60. Pub Med PMID: 22537354.
33
Highlights
PREVENTING
Atrial Fibrillation Related
STROKES with Anticoagulants
• Prevalence and incidence of AF
• Risk stratification for stroke and bleeding
• New oral anticoagulants
• Guidelines
• Practical considerations for choosing an anticoagulant
Question #5
78 year old female with atrial fibrillation, hypertension and
CHF.
CHADS
2
= 3
CHA
2
DS
2
-VASc = 5
HAS-BLED = 2
What would you use for stroke prevention?
1.
No anti-thrombotics
2.
Aspirin
3.
4.
5.
Aspirin + clopidogrel
VKA antagonist
Dabigatran or Rivaroxaban
35
European Society of Cardiology Guidelines
CHA
2
DS
2
-VASc and Stroke Rate
Risk Factors
For Stroke and Thrombo-embolism in Non-valvular AF
Risk Factor Score
C ongestive heart failure/LV dysfunction*
H ypertension*
A ge >75**
D iabetes Mellitus*
S troke / TIA / Thrombo-embolism**
V ascular Disease*
A ge 65-74*
S ex category (i.e. female sex)*
Maximum Score
Note: maximum score is 9 since age may contribute 0,1, or 2 points
* ‘Clinically relevant non-major’ risk factor
** “Major” risk factor
1
9
1
1
2
1
1
1
2
Camm AJ. Europace. 2010 Oct;12(10):1360-420. Pub Med PMID: 20876603.
36
European Society of Cardiology Guidelines
Approach to Thromboprophylaxis in Patients with AF
Risk Category
One ‘major’ risk factor or > 2 ‘clinically relevant nonmajor’ risk factors
One ‘clinically relevant nonmajor’ risk factor’
CHA
2
DS
2
-VASc
Score
> 2
1
No risk factors 0
Recommended
Antithrombotic Therapy
OAC
1
• Either OAC or aspirin 75-325 mg daily
• Preferred: OAC rather than aspirin
• Either aspirin 75-325 mg daily or no antithrombotic therapy
• Preferred: no antithrombotic therapy rather than aspirin
Risk of Bleeding
Low risk
Measurable risk, or 1 clinicallyrelevant non-major risk factor
HAS-BLED Score Dabigatran Dosage 2
0 –2
≥3
1. Camm AJ. Europace. 2010 Oct;12(10):1360-420. Pub Med PMID: 20876603.
2. Connolly SJ, et al. N Engl J Med 2009;361:1139 –1151. PMID: 19717844.
150 mg b.i.d.
110 mg b.i.d.
37
2011 ACCF/AHA/HRS Guidelines
Antithrombotic Therapy for Patients with Atrial Fibrillation
Risk Category
1
No risk factors
Recommended Therapy
Aspirin, 81 to 325 mg daily
One moderate risk factor Aspirin, 81 to 325 mg daily, or warfarin (INR 2.0 to 3.0, target 2.5)
Any high risk factor or
> 1 moderate-risk factor
Warfarin (INR 2.0 to 3.0, target 2.5)*
Less Validated /
Weaker Risk Factors
Female gender
Age 65 to 74 years
1
Coronary artery disease
Thyrotoxicosis
Moderate Risk Factors
Age >75 years
Hypertension
Heart failure
LV ejection fraction <35%
Diabetes mellitus
High Risk Factors
Previous stroke, TIA or embolism
Mitral stenosis
Prosthetic heart valve*
* If mechanical valve, target international normalized ratio (INR) > 2.5
2011 Focused Update Recommendation Class I
2
Dabigatran is useful as an alternative to warfarin for the prevention of stroke and systemic thromboembolism in patients with paroxysmal to permanent AF and risk factors for stroke or systemic embolization who do not have a prosthetic heart valve or hemodynamically significant valve disease, severe renal failure (creatinine clearance <15 mL/min) or advanced liver disease
(impaired baseline clotting function).
(Level of Evidence: B)
1. Fuster V. Circulation. 2011 Mar 15;123(10): Pub Med PMID: 21382897.
2. Wann LS, et al. J Am Coll Cardiol. 2011 Mar 15;57(11):1330-7. Pub Med PMID: 21324629.
Comments
New Recommendation
38
ACCP Guidelines
For patients with Nonrheumatic AF, including those with Paroxysmal AF
Level of Risk
Low Risk
(CHADS
2
= 0)
Intermediate Risk
(CHADS
2
= 1)
High Risk
(CHADS
2
= 2)
ACCP
Recommendation Alternative* Not Recommended
No Therapy Aspirin
Oral anticoagulation Aspirin with clopidogrel
Oral anticoagulation or combination therapy with aspirin and clopidogrel
Aspirin
Oral anticoagulation
(dabigatran 150 mg b.i.d. vs. VKA**)
Aspirin with clopidogrel
Aspirin
*For patients with AF unsuitable for, or who refuse, oral anticoagulant (for reasons other than concerns about major bleeding)
**VKA = adjusted-dose vitamin K antagonist
You JJ, et al. Chest. 2012 Feb;141(2 Suppl):e531S-75S. Pub Med PMID: 22315271.
39
Canadian Cardiovascular Society
Guidelines
Assess Thromboembolic Risk
(CHADS
2
)
CHADS
2
= 1 CHADS
2
= 2
Increasing stroke risk
OAC* OAC or vascular
Age > 65 yrs or combination female sex and disease
*ASA is a reasonable indicated by risk/benefit
When OAC therapy is indicated, most patients receive:
• Dabigatran, rivaroxaban, or apixaban (after Health
Canada approval)
• In preference to warfarin
• Conditional Recommendation,
High-Quality Evidence
Skanes AC, et al. Can J Cardiol. 2012 Mar-Apr;28(2):125-36. Pub Med PMID: 22433576.
40
Highlights
PREVENTING
Atrial Fibrillation Related
STROKES with Anticoagulants
• Prevalence and incidence of AF
• Risk stratification for stroke and bleeding
• New oral anticoagulants
• Guidelines
• Practical considerations for choosing an anticoagulant
Optimal Candidates for New Drugs
Patients who:
• Find INR testing burdensome
• Despite adherence to provider recommendations, have low ‘time-in-range’
• Can afford (or arrange to get) the new drugs
• Have normal renal function
42
Optimal Candidates for Warfarin
Patients who:
• Have (borderline) renal insufficiency
• Are taking stable dose of warfarin and do not find INR testing burdensome
• Have access to self-testing machine
• Are concerned about the lack of an evidence-based reversal strategy
43
TTR per Country in RELY
90
80
70
60
50
44
47 48 49 49
53 53 54 55 55
56 56 56 57 58 58
60 60 62 62
64 64 64 64 64 65 65 66 66 66
67 68 68
70 70 70 71 71 72 72 72
74 74
77
40
30
20
10
0
Wallentin L, et al. Lancet. 2010 Sep 18;376(9745):975-83. PMID: 20801496.
USA:
Improvement
Needed
44
Stroke and Systemic Embolism
By Center TTR in RELY
Wallentin L, et al. Lancet. 2010 Sep 18;376(9745):975-83. Pub Med PMID: 20801496.
• TTR=optimum therapeutic range
• cTTR=center's mean TTR
45
Major Bleeding
By Center TTR in RELY
Wallentin L, et al. Lancet. 2010 Sep 18;376(9745):975-83. PMID: 20801496.
• TTR=optimum therapeutic range
• cTTR=center's mean TTR
46
Stroke and Systemic Embolization by
Center Proportion of INR in Therapeutic
Range in ROCKET AF
Center TTR‡
Rivaroxaban
Total
45/1735 (2.59)
Event Rate
(100 Pt Yrs) §
1.77
Total
Warfarin
62/1689 (3.67)
Event Rate
(100 Pt Yrs) §
2.53
0.00-50.6%
50.7%-58.5% 53/1746 (3.04)
54/1734 (3.11)
1.94
1.90
63/1807 (3.49)
62/1758 (3.53)
2.18
2.14
58.6-65.7%
65.7-100.0% 37/1676 (2.21) 1.33
55/1826 (3.01)
N=7061 rivaroxaban N=7082 warfarin
P value for interaction=0.736
Time in therapeutic range-2-3 inclusive
‡Center TTR calculated using total INR values in target range from all warfarin subjects within center, divided by total INR values from all warfarin subjects within center
§Number of events per 100 patient-years of follow-up
II Hazard ratio from Cox proportional hazard model with treatment as a covariate
1.80
Rivaroxaban vs. Warfarin
Hazard Ratio
(95% CI)II
0.70 (0.48, 1.03)
0.89 (0.62, 1.29)
0.89 (0.62, 1.28)
0.74 (0.49, 1.12)
Patel MR, et al. N Engl J Med. 2011 Sep 8;365(10):883-91. Pub Med PMID: 21830957.
47
Summary
PREVENTING
Atrial Fibrillation Related
STROKES with Anticoagulants
• Prevalence and incidence of AF
• Risk stratification for stroke and bleeding
• New oral anticoagulants
• Guidelines
• Practical considerations for choosing an anticoagulant
48