Are Most Published Research Findings False?
advertisement

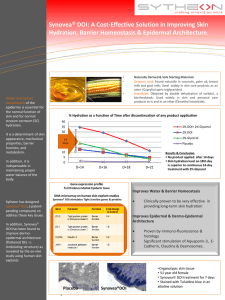
ARE MOST PUBLISHED RESEARCH FINDINGS FALSE? IMPLICATIONS FOR PRACTICING “EVIDENCE-BASED MEDICINE” Sheila Modi Best Practices Conference July 31, 2013 Ioannides “There is increasing concern that most current published research findings are false.” “Simulations show that for most study designs and settings, it is more likely for a research claim to be false than true.” “Moreover, for many current scientific fields, claimed research findings may often be simply accurate measures of the prevailing bias.” Ioannidis JPA (2005) Why Most Published Research Findings Are False. PLoS Med 2(8): e124. doi:10.1371/journal.pmed.0020124 http://www.plosmedicine.org/article/info:doi/10.1371/journal.pmed.0020124 Ioannidis - proof “It can be proven that most claimed research findings are false.” Formal statistical significance claimed on basis of p<0.05. After a study has generated a statistically significant positive finding, then the post-study probability that finding is true is the PPV (positive predictive value). R (pre-study odds). The higher the R, higher the PPV. Bias (u). With increasing bias, the PPV decreases. More independent teams studying the same thing (n studies), PPV decreases. The one study achieving significant findings often receives unilateral attention. Ioannidis JPA (2005) Why Most Published Research Findings Are False. PLoS Med 2(8): e124. doi:10.1371/journal.pmed.0020124 http://www.plosmedicine.org/article/info:doi/10.1371/journal.pmed.0020124 Ioannidis’s corollaries Corollary 1. The smaller the studies (less sample size, less power), the less likely research findings are true. Corollary 2. The smaller the effect sizes, the less likely research findings are true. Corollary 3. The greater the number and the lesser the selection of tested relationships, the less likely research findings are true. Corollary 4. The greater the flexibility in designs, definitions, outcomes, and analytical modes, the less likely research findings are true. Corollary 5. The greater the financial and other interests and prejudices, the less likely research findings are true. Ioannidis JPA (2005) Why Most Published Research Findings Are False. PLoS Med 2(8): e124. doi:10.1371/journal.pmed.0020124 http://www.plosmedicine.org/article/info:doi/10.1371/journal.pmed.0020124 Table 4. PPV of Research Findings for Various Combinations of Power (1 - ß), Ratio of True to Not-True Relationships (R), and Bias (u) Ioannidis JPA (2005) Why Most Published Research Findings Are False. PLoS Med 2(8): e124. doi:10.1371/journal.pmed.0020124 http://www.plosmedicine.org/article/info:doi/10.1371/journal.pmed.0020124 Ok, but we already knew this… EBM Notebook: Improving journal club presentations, or, I can present that paper in under 10 minutes 6. State your answers to the critical appraisal questions on validity Did the experimental and control groups start out with a similar prognosis? – Were patients randomized? YES. – Was randomisation concealed? YES. – Were patients analysed in the groups to which they were randomised? YES— intention to treat analysis. – Were groups similar re known prognostic factors? YES—see table 1. Did the experimental and control groups retain a similar prognosis after the study started? – Were patients, clinicians, and outcome assessors aware of group allocation? NO—all were blinded to random allocation. – Was follow up complete? YES and similar in each group. 8. Describe why you think the results can or cannot be applied to your patients/situation. Schwartz MD, Dowell D, Aperi J, Kalet AL. Improving journal club presentations, or, I can present that paper in under 10 minutes. Evid Based Med. 2007 Jun;12(3):66-8. PubMed ID: 17537877. Bias Selective or distorted reporting Financial conflict of interest Using “hard” (e.g. death) vs. “soft” (e.g. markers) outcomes Asking a biased question (e.g. comparing a new drug to a drug already proven to be inferior to other drugs on market) Researchers/academics focus on obtaining funding (researchers tend to find whatever results are most likely to get them funded) Researchers/academics also focus on getting published. Well-regarded journals have a rejection rate of 90%. Extravagant claims are more likely to get published. Verifying anothers’ results is less likely to get published. Researchers develop personal investment in a field or a finding “Hype” on a topic Inadequate safety data Ioannidis JPA (2005) Why Most Published Research Findings Are False. PLoS Med 2(8): e124. doi:10.1371/journal.pmed.0020124 http://www.plosmedicine.org/article/info:doi/10.1371/journal.pmed.0020124 Ioannidis “Published research findings are sometimes refuted by subsequent evidence, with ensuing confusion and disappointment.” Ioannidis JPA (2005) Why Most Published Research Findings Are False. PLoS Med 2(8): e124. doi:10.1371/journal.pmed.0020124 http://www.plosmedicine.org/article/info:doi/10.1371/journal.pmed.0020124 David Sackett David Sackett, often referred to as the “father of evidence-based medicine,” once famously said: “Half of what you’ll learn in medical school will be shown to be either dead wrong or out of date within five years of your graduation; the trouble is that nobody can tell you which half–so the most important thing to learn is how to learn on your own.” http://retractionwatch.wordpress.com/2011/07/11/so-how-often-does-medical-consensus-turn-out-to-be-wrong/ Refuted research Study (Ioannidis, 2005) evaluated papers published in major journals and cited >1000 times in the previous 13 years. (“High impact papers”) Found 49 studies, of which 45 claimed an intervention was effective. 7 (16%) were contradicted by subsequent studies. 7 (16%) found effects that were stronger than those shown in subsequent studies. 20 (44%) were replicated. 11 (24%) remained unchallenged. Subgroups: 5 of 6 non-randomized studies were contradicted or had found stronger effects vs. 9 of 39 RCTs (p = 0.008). Among RCTs, smaller studies (smaller n) were more likely to be contradicted or had found stronger effects (p = 0.009). Ioannidis JA. Contradicted and Initially Stronger Effects in Highly Cited Clinical Research. JAMA. 2005;294(2):218-228. doi:10.1001/jama.294.2.218. Refuted research- another study Study (Prasad, 2011) looked at all studies in the New England Journal of Medicine (NEJM) in 2009 There were 124 studies that “made some claim with respect to a medical practice.” 89 (72%) investigated a new medical practice; 25 (28%) studies a practice already in adoption 91 (78%) were RCTs; 19 (15%) were prospective cohort studies; 13 (10%) were retrospective cohort; 1 was a case-control study. 82 (66%) reported positive findings; 42 (33%) reported negative findings 61 (49%) reported a new practice surpassing current care; 12 (10%) reported a new practice failing to improve on current practice; 16 (13%) reported an existing practice that was upheld as beneficial; and 16 (13%) constituted reversals. 19 (15%) were classified as inconclusive. Prasad V, Gall V, Cifu A. The Frequency of Medical Reversal. Arch Intern Med. 2011;171(18):1675-1676. doi:10.1001/archinternmed.2011.295. Refuted research- another study Study (Prasad, 2013) looked at all 2044 articles published in NEJM from 2001-2010. 1344 articles concerned a medical practice. Of these: 981articles (73.0%) examined a new medical practice, whereas 363 (27.0%) tested an established practice. A total of 947 studies (70.5%) had positive findings, whereas 397 (29.5%) reached a negative conclusion. A total of 756 articles addressing a medical practice constituted replacement, 165 were back to the drawing board, 146 were medical reversals, 138 were reaffirmations, and 139 were inconclusive. Of the 363 articles testing standard of care, 146 (40.2%) reversed that practice, whereas 138 (38.0%) reaffirmed it, 79 (27.3%) were inconclusive. Articles that tested new practices were more likely to find them beneficial than articles that tested existing ones (77.1% vs 38.0%; P<.001). Conversely, articles that tested existing standards were more likely to find those practices ineffective than articles testing new practices (40.2% vs 17.0%; P<.001). Prasad V, et. al. A Decade of Reversal: An Analysis of 146 Contradicted Medical Practices. Mayo Clin Proc. Aug 2013; 1-9. n http://dx.doi.org/10.1016/j.mayocp.2013.05.012. Prasad, 2013 “Many reversals have similar narratives. Although there is a weak evidence base for some practice, it gains acceptance largely through vocal support from prominent advocates and faith that the mechanism of action is sound. Later, future trials undermine the therapy, but removing the contradicted practice often proves challenging.” Prasad V, et. al. A Decade of Reversal: An Analysis of 146 Contradicted Medical Practices. Mayo Clin Proc. Aug 2013; 1-9. n http://dx.doi.org/10.1016/j.mayocp.2013.05.012. Prasad, 2013 “Recently, a project of BMJ, entitled Clinical Evidence, completed a review of 3000 medical practices. The project found that: slightly more than a third of medical practices are effective or likely to be effective; 15% are harmful, unlikely to be beneficial, or a tradeoff between benefits and harms; and 50% are of unknown effectiveness. Our investigation complements these data and suggests that a high percentage of all practices may ultimately be found to have no net benefits.” Prasad V, et. al. A Decade of Reversal: An Analysis of 146 Contradicted Medical Practices. Mayo Clin Proc. Aug 2013; 1-9. n http://dx.doi.org/10.1016/j.mayocp.2013.05.012. Persistence of wrong findings Even when a research finding is refuted, that idea typically persists for years or even decades. Study (Tatsioni, 2007) looked at 2 highly cited studies citing evidence for vitamin E’s cardiovascular benefits from 1993 (refuted in 2005). Also looked at articles citing evidence for b-carotene in cancer from 1981 (refuted in 1994-1996) Also looked at articles citing evidence for estrogen in Alzheimer’s disease from 1996 (refuted 2004). Found that researchers cited the original results as correct more often than as wrong, even after a number of years. Tatsioni A, Bonitsis NG, Ioannidis JA. Persistence of Contradicted Claims in the Literature. JAMA. 2007;298(21):2517-2526. doi:10.1001/jama.298.21.2517. Harm to patients Not only when we give interventions to patients that may not help them, but also those interventions often cause additional harm. Steen writes about “harm by influence” • http://retractionwatch.wordpress.com/2011/06/13/no-academic-matter-study-links-retractions-to-patient-harm/ • Steen RG. Retractions in the medical literature: how many patients are put at risk by flawed research? J Med Ethics 2011;37:688-692 doi:10.1136/jme.2011.043133 Safety Often clinical trials are not long enough to find adverse effects of medications These are often found later, after medications or procedures have been accepted in general use. Often clinical trials study a drug in isolation, and there is little data on combinations of drugs that result in adverse effects. Google A study (White, 2013) used data drawn from queries entered into Google, Bing, and Yahoo search engines in 2010 They looked for searches relating to paroxetine and pravastatin and symptoms of hyperglycemia. They were able to find evidence that the combination of the two drugs caused high blood sugar. These searches were done before this adverse effect was found by the Food and Drug Administration’s warning system. • Markoff J. (2013, March 6). Unreported side effects of drugs are found using internet search data, study finds. The New York Times. http://www.nytimes.com/2013/03/07/science/unreported-side-effects-of-drugs-found-using-internet-data-study-finds.html?_r=0 • White RW, et. al. Web-scale pharmacovigilance: listening to signals from the crowd. J Am Med Inform Assoc. 2013 May 1;20(3):404-8. doi: 10.1136/amiajnl-2012-001482. Epub 2013 Mar 6. Januvia (sitagliptin) • • Sitagliptin = dipeptidyl peptidase4 inhibitor (increases GLP-1), incretin effect -> stimulates insulin release from pancreatic cells. Merck’s Januvia and the related Janumet (Januvia+metformin) had global sales of $5.7 billion last year. Safety concerns: There are 43 lawsuits which claim that Januvia caused pancreatic cancer, according to Merck. Pollack A. (2013, May 30). A Lone Voice Raises Alarms on Lucrative Diabetes Drugs. The New York Times. http://www.nytimes.com/2013/05/31/business/a-doctor-raises-questions-about-adiabetes-drug.html?pagewanted=all&_r=0 http://www.uptodate.com.libproxy.unm.edu/contents/glucagon-like-peptide-1-based-therapies-for-the-treatment-of-type-2-diabetesmellitus?detectedLanguage=en&source=search_result&search=sitagliptin&selectedTitle=5~23&provider=noProvider#H13 Januvia harm studies A study (Elashoff, 2011) looked at FDA’s database of reported adverse events on sitagliptin and exenatide 2004-2009 compared with 4 control medications. Use of sitagliptin or exenatide increased the odds ratio for reported pancreatitis 6-fold as compared with other therapies (p < 2 x 10-16). Pancreatic cancer was more commonly reported among patients who took sitagliptin or exenatide as compared with other therapies (p < 0.008, p < 9 x 105). All other cancers occurred similarly among patients who took sitagliptin compared with other therapies p= 0.20). • Elashoff M, et. al. Pancreatitis, Pancreatic, and Thyroid Cancer With Glucagon-Like Peptide-1–Based Therapies. Gastroenterology, Volume 141, Issue 1, July 2011, Pages 150–156. http://dx.doi.org.libproxy.unm.edu/10.1053/j.gastro.2011.02.018 Januvia harm studies (cont’d) A study (Butler, 2013) looked at cadaver pancreases of T2DM pts who had incretin therapy or other therapy compared to non-diabetic controls. • • • ∼40% increased pancreatic mass in DM treated with incretin therapy, with both increased exocrine cell proliferation (P < 0.0001) and dysplasia (increased pancreatic intraepithelial neoplasia, P < 0.01). Pancreata in DM treated with incretin therapy were notable for α-cell hyperplasia and glucagon-expressing microadenomas (3 of 8) and a neuroendocrine tumor. β-Cell mass was reduced by ∼60% in those with DM, yet a sixfold increase was observed in incretin-treated subjects, although DM persisted. Endocrine cells co-staining for insulin and glucagon were increased in DM compared with non-DM control subjects (P < 0.05) and markedly further increased by incretin therapy (P < 0.05). In conclusion, incretin therapy in humans resulted in a marked expansion of the exocrine and endocrine pancreatic compartments, the former being accompanied by increased proliferation and dysplasia and the latter by α-cell hyperplasia with the potential for evolution into neuroendocrine tumors. A study (Gier, 2012) looked at GLP-1 analog exendin-4 in rats, mice, & human pancreatic duct cells Treatment of rats for 12 weeks induced a marked expansion of pancreatic duct glands (PDGs) through the mechanism of enhanced PDG cell replication. Moreover, we report that the PDGs in rats and humans express GLP-1Rs and that these also are abundantly expressed in pancreatic intraepithelial neoplasia (PanIN) lesions in human pancreas. Treatment in a mouse model prone to the development of pancreatic ductal adenocarcinoma for 12 wks advances the rate of formation of dysplastic mPanIN lesions and chronic pancreatitis. Treatment of human pancreatic duct cells (in vitro) with the GLP-1 analog exendin-4 induces proproliferative signaling pathways, an effect that is inhibited by metformin. Collectively, these studies imply that GLP-1–induced proliferation within the exocrine pancreas is focal and may accelerate the development of dysplastic lesions when present. A study (Matveyenko, 2009) looked at sitagliptin and metformin in rats for 12 wks The combination of metformin and sitagliptin had synergistic actions to preserve β-cell mass and function and enhance insulin sensitivity in the HIP rat model of type 2 diabetes. Sitagliptin treatment was associated with increased pancreatic ductal turnover, ductal metaplasia, and, in one rat, pancreatitis. Matveyenko AV, et. al. Beneficial Endocrine but Adverse Exocrine Effects of Sitagliptin in the Human Islet Amyloid Polypeptide Transgenic Rat Model of Type 2 Diabetes: Interactions With Metformin. Diabetes 58:1604–1615, 2009 Butler AE, et. al. Marked Expansion of Exocrine and Endocrine Pancreas With Incretin Therapy in Humans With Increased Exocrine Pancreas Dysplasia and the Potential for Glucagon-Producing Neuroendocrine Tumors. Diabetes July 2013 vol. 62 no. 7 2595-2604. Gier B. Chronic GLP-1 Receptor Activation by Exendin-4 Induces Expansion of Pancreatic Duct Glands in Rats and Accelerates Formation of Dysplastic Lesions and Chronic Pancreatitis in the KrasG12D Mouse Model. Diabetes May 2012 vol. 61 no. 5 1250-1262. Januvia- Merck study • • Merck data: Comined data from 14,611 patients in 25 studies (all randomized, double-blind trials conducted by Merck) with T2DM who received either sitagliptin 100 mg/day (n = 7,726; sitagliptin group) or a comparator agent (n = 6,885; nonexposed group). Treatment duration: between 12 weeks and 2 years These studies assessed sitagliptin, versus comparator agents, taken as monotherapy, initial combination therapy with metformin or pioglitazone, or as add-on combination therapy with other antihyperglycemic agents (metformin, pioglitazone, a sulfonylurea ± metformin, insulin ± metformin, or metformin + pioglitazone or rosiglitazone). Patient-level data from each study were used to evaluate between-group differences in the exposure-adjusted incidence rates of adverse events (AEs). Results: Overall incidence rates of AEs and drug-related AEs were higher in the non-exposed group compared with the sitagliptin group. Incidence rates of specific AEs were generally similar between the two groups, except for higher incidence rates of hypoglycemia related to the greater use of a sulfonylurea and diarrhea related to the greater use of metformin in the non-exposed group, and of constipation in the sitagliptin group. Treatment with sitagliptin was not associated with an increased risk of major adverse cardiovascular events, malignancy, or pancreatitis. Nancy Thornberry, who heads diabetes drug development at Merck, said that clinical trials, the gold standard of medical evidence, had found no increased risk of pancreatic disease from Januvia, even when results of trials were pooled to achieve greater numbers. “In fact, my mother takes sitagliptin,” she added. Engel SS, et. al. Safety and Tolerability of Sitagliptin in Type 2 Diabetes: Pooled Analysis of 25 Clinical Studies. Diabetes Therapy. June 2013, Volume 4, Issue 1, pp 119-145. Pollack A. (2013, May 30). A Lone Voice Raises Alarms on Lucrative Diabetes Drugs. The New York Times. http://www.nytimes.com/2013/05/31/business/a-doctor-raises-questions-about-a-diabetes-drug.html?pagewanted=all&_r=0 One step further A small point about Statins. Statins Proven mortality benefit in large, RCTs 4S trial 1994 showed in pts with CAD, simvastatin: Reduced total cholesterol, reduced LDL, increased HDL Reduced death (total mortality) (RR death in simva group 0.70 -> NNT 30) Reduced coronary death (RR 0.58) A recent meta-analysis of 14 statin trials (using lovastatin, simvastatin, pravastatin, fluvastatin, or atorvastatin) with total 90,056 pts randomized showed that: The decrease in coronary events was best predicted by the absolute decrease in LDL levels. The incidence of major vascular events was reduced by ∼20% for each 40 mg/dl decrease in LDL cholesterol42. Thus, an individual starting with an LDL of 280 mg/dl whose level decreased to 200 mg/dl on therapy (a 29% decrease) would be predicted to have a 40% decrease in risk over a 5 year period. • Pederson TR, et. al. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). 1994. Atheroscler Suppl. 2004 Oct;5(3):81-7. • Steinberg D. Thematic review series: the pathogenesis of atherosclerosis. An interpretive history of the cholesterol controversy, part V: the discovery of the statins and the end of the controversy. J Lipid Res. 2006 Jul;47(7):1339-51. Epub 2006 Apr 3. Physical fitness and Mortality Meta-analysis, 33 eligible studies, of healthy pts Pooled RRs of all-cause mortality and CHD/CVD events per 1MET higher level of MAC (corresponding to 1-km/h higher running/jogging speed) were: All-cause mortality: RR 0.87 (95% confidence interval [CI], 0.84-0.90) CHD/CVD events RR 0.85 (95% CI, 0.82-0.88), respectively. Participants were categorized as low CRF (<7.9 METs), intermediate CRF (7.9- 10.8 METs), or high CRF (>10.9 METs). Compared with participants with high CRF, those with low CRF had: RR for all-cause mortality of 1.70 (95% CI, 1.51-1.92; P < .001) RR for CHD/CVD events of 1.56 (95% CI, 1.39-1.75; P< .001), adjusting for heterogeneity of study design. Compared with participants with intermediate CRF, those with low CRF had: RR for all-cause mortality of 1.40 (95% CI, 1.32-1.48; P<.001) RR for CHD/CVD events of 1.47 (95% CI, 1.35-1.61; P<.001), adjusting for heterogeneity of study design. Conclusions: Better CRF was associated with lower risk of allcause mortality and CHD/CVD. Participants with a MAC of 7.9 METs or more had substantially lower rates of all-cause mortality and CHD/CVD events compared with those with a MAC of less 7.9 METs. Kodama S, et. al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009 May 20;301(19):2024-35. doi: 10.1001/jama.2009.681. METs (VO2 / 3.5 = METs) Fleisher LA, et. al. ACC/AHA 2007 Guidelines on Perioperative Cardiovascular Evaluation and Care for Noncardiac Surgery A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). Circulation. 2007;116:e418-e500; originally published online September 27, 2007; doi: 10.1161/CIRCULATIONAHA.107.185699. Statins and VO2 max Thirty-seven participants (exercise plus statins; n=18; exercise only; n=19) completed the 12 week study (non-blinded). Anthropormorphic outcomes: Body weight decreased significantly in the exercise group (Table 1; P<0.01 for change within group) but not the exercise plus statin group (P<0.01 for between-group difference in change from baseline). Similarly, there was a significant decrease in fat mass in the exercise group (P<0.05). In the exercise plus statin group, the decrease in fat mass approached significance (P=0.056). Lean body mass increased significantly in the exercise plus statin group only (P<0.05 for with-in group change from baseline; P<0.05 for difference in between-group change from baseline) Lipids: Total cholesterol decreased by 29% (P<0.001 for within-group change from baseline) and LDL decreased by 38% (P<0.001) in the exercise plus statin group. There were no significant changes in total cholesterol or LDL in the exercise group (P<0.001 for between-group differences in change from baseline). HDL did not change significantly in either group. Cardiorespiratory fitness: increased by 10% (P<0.05) in response to exercise training alone, but was blunted by the addition of simvastatin resulting in only a 1.5% increase (P<0.005 for group by time interaction). Similarly, skeletal muscle citrate synthase activity increased by 13% in the exercise only group (P <0.05), but decreased by 4.5% in the simvastatin plus exercise group (P<0.05 for group by time interaction). CONCLUSION: Simvastatin attenuates increases in cardiorespiratory fitness and skeletal muscle mitochondrial content when combined with exercise training in overweight or obese patients at risk of the metabolic syndrome. • Reynolds G. (2013, May 22). Can statins cut the benefits of exercise? The New York Times.http://well.blogs.nytimes.com/2013/05/22/canstatins-curb-the-benefits-of-exercise/ • Mikus CR, Boyle LJ, Borengasser SJ, Oberlin DJ, Naples SP, Fletcher, J, Meers GM, Ruebel M, Laughlin MH, Dellsperger KC, Fadel PJ, Thyfault JP, Simvastatin impairs exercise training adaptations, Journal of the American College of Cardiology (2013), doi: 10.1016/j.jacc.2013.02.074. Statins vs. VO2 max • • • Statins: Reduce death (all-cause) RR 0.70 and cardiovascular death RR 0.58. Every 1 millimole per liter (40 mg/dl) reduction in LDL is associated with a 10-20% reduction in risk of cardiovascular events and all-cause mortality Exercise: Increasing aerobic fitness by 1 MET reduces death RR 0.87. Every 1 MET (3.5 milliters of oxygen per kilogram of body weight per minute) increase in fitness is associated with an 18% reduction in cardiovascular disease mortality and an 1150% reduction in all-cause mortality. Low aerobic fitness compared to high aerobic fitness RR death 1.70 and cardiovascular death 1.56. Pederson TR, et. al. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). 1994. Atheroscler Suppl. 2004 Oct;5(3):81-7. Steinberg D. Thematic review series: the pathogenesis of atherosclerosis. An interpretive history of the cholesterol controversy, part V: the discovery of the statins and the end of the controversy. J Lipid Res. 2006 Jul;47(7):1339-51. Epub 2006 Apr 3. Mikus CR, Boyle LJ, Borengasser SJ, Oberlin DJ, Naples SP, Fletcher, J, Meers GM, Ruebel M, Laughlin MH, Dellsperger KC, Fadel PJ, Thyfault JP, Simvastatin impairs exercise training adaptations, Journal of the American College of Cardiology (2013), doi: 10.1016/j.jacc.2013.02.074. Statins vs VO2 max (cont’d) • • • Statins and exercise: A study showed, as cardiorespiratory fitness increases, the predictive value of LDL on coronary heart disease mortality is significantly attenuated in men. In a large, prospective study of dyslipidemic veterans, both fitness and statin use were independently associated with low mortality, with the lowest risk of mortality observed in highly fit patients taking statins. Notably, patients in the highest quartile of fitness had a 60-70% reduction in all-cause mortality relative to patients in the lowest quartile of fitness, irrespective of statin use, and the low-fit patients taking statins had a higher risk of mortality than highly fit patients not taking statins. “There are NO randomized, placebo-controlled trials directly comparing the longterm cardio-protective effects of exercise alone to statins plus exercise. Until such studies are undertaken, the relative importance of improving fitness and lowering LDL in moderating risk of cardiovascular events and death should be carefully weighed in the clinical setting.” Pederson TR, et. al. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). 1994. Atheroscler Suppl. 2004 Oct;5(3):81-7. Steinberg D. Thematic review series: the pathogenesis of atherosclerosis. An interpretive history of the cholesterol controversy, part V: the discovery of the statins and the end of the controversy. J Lipid Res. 2006 Jul;47(7):1339-51. Epub 2006 Apr 3. Mikus CR, Boyle LJ, Borengasser SJ, Oberlin DJ, Naples SP, Fletcher, J, Meers GM, Ruebel M, Laughlin MH, Dellsperger KC, Fadel PJ, Thyfault JP, Simvastatin impairs exercise training adaptations, Journal of the American College of Cardiology (2013), doi: 10.1016/j.jacc.2013.02.074. Statins vs VO2 max (cont’d) Also, this does not take into account the previously mentioned drug-drug interaction between statin + paroxetine that causes diabetes (diabetes is known to increase risk of death and cardiovascular death) Also of note, exercise has also been shown to effectively treat depression Maybe we don’t really need either statins or SSRIs? Another study looked at the Mediterranean diet and CV disease… Why can’t we write an Rx for exercise and a salad? Cost of simvastatin (generic) ~$5 per pill ($150/month = $1800/year) Merck’s Zocor sales of $3.3 billion in 2005 in the U.S., and $4.4 billion worldwide Then on June 23,2006, patent expired, so revenues fell Pfizer’s Lipitor sales totaled $8 billion in 2005 in the U.S., the “most profitable medicine yet invented” The valuation of the worldwide market for all statins was $19.7 billion in 2012 New Mexico ABQ YMCA gym membership $50/month = $600/year. >>Sheila’s opinion: The prevailing attitude “There’s a pill for that” comes from effective marketing! • http://www.cardiovascularbusiness.com/topics/healthcare-economics/slides-generics-push-statin-revenues-down-7b • http://www.uptodate.com.libproxy.unm.edu/contents/simvastatin-drug-information detectedLanguage=en&source=search_result&search=simvastatin&selectedTitle=1~144&provider=noProvider#F221287 • Berensen, A.(2006, June 23). Merck loses protection for patent on Zocor. The New York Times. http://www.nytimes.com/2006/06/23/business/23statin.html • http://ymcacnm.org/join/membership-rates/ Bias Modern medicine and much medical research is biased towards pharmaceutical or procedural answers to our health problems because of money. Conclusions Most published research is false. Money = bias. All drugs have side effects. We probably don’t know about them all, and they may be pretty serious. “Less is More” (Archives of Internal Medicine), “Choosing Wisely” (ABIM) References (1) Gutting G. (2013, April 25). What do scientific studies show? The New York Times. http://opinionator.blogs.nytimes.com/2013/04/25/what-doscientific-studies-show/?emc=eta1 Freedman DH. (2010, October 4). Lies, damned lies, and medical science. The Atlantic. http://www.theatlantic.com/magazine/archive/2010/11/liesdamned-lies-and-medical-science/308269/ Ioannides JPA. (2005). Why most published research findings are false. PLoS Med 2(8): e124. doi:10.1371/journal/pmed.0020124. Schwartz MD, Dowell D, Aperi J, Kalet AL. Improving journal club presentations, or, I can present that paper in under 10 minutes. Evid Based Med. 2007 Jun;12(3):66-8. PubMed ID: 17537877. Ioannidis JA. Contradicted and Initially Stronger Effects in Highly Cited Clinical Research. JAMA. 2005;294(2):218-228. doi:10.1001/jama.294.2.218. Prasad V, Gall V, Cifu A. The Frequency of Medical Reversal. Arch Intern Med. 2011;171(18):1675-1676. doi:10.1001/archinternmed.2011.295. Prasad V, et. al. A Decade of Reversal: An Analysis of 146 Contradicted Medical Practices. Mayo Clin Proc. Aug 2013; 1-9. n http://dx.doi.org/10.1016/j.mayocp.2013.05.012. Tatsioni A, Bonitsis NG, Ioannidis JA. Persistence of Contradicted Claims in the Literature. JAMA. 2007;298(21):2517-2526. doi:10.1001/jama.298.21.2517. http://retractionwatch.wordpress.com/2011/07/11/so-how-often-does-medical-consensus-turn-out-to-be-wrong/ Markoff J. (2013, March 6). Unreported side effects of drugs are found using internet search data, study finds. The New York Times. http://www.nytimes.com/2013/03/07/science/unreported-side-effects-of-drugs-found-using-internet-data-study-finds.html?_r=0 White RW, et. al. Web-scale pharmacovigilance: listening to signals from the crowd. J Am Med Inform Assoc. 2013 May 1;20(3):404-8. doi: 10.1136/amiajnl-2012-001482. Epub 2013 Mar 6. http://retractionwatch.wordpress.com/2011/06/13/no-academic-matter-study-links-retractions-to-patient-harm/ Steen RG. Retractions in the medical literature: how many patients are put at risk by flawed research? J Med Ethics 2011;37:688-692 doi:10.1136/jme.2011.043133 Reynolds G. (2013, May 22). Can statins cut the benefits of exercise? The New York Times.http://well.blogs.nytimes.com/2013/05/22/can-statinscurb-the-benefits-of-exercise/ Mikus CR, Boyle LJ, Borengasser SJ, Oberlin DJ, Naples SP, Fletcher, J, Meers GM, Ruebel M, Laughlin MH, Dellsperger KC, Fadel PJ, Thyfault JP, Simvastatin impairs exercise training adaptations, Journal of the American College of Cardiology (2013), doi: 10.1016/j.jacc.2013.02.074. Kodama S, et. al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009 May 20;301(19):2024-35. doi: 10.1001/jama.2009.681. References (2) Fleisher LA, et. al. ACC/AHA 2007 Guidelines on Perioperative Cardiovascular Evaluation and Care for Noncardiac Surgery A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). Circulation. 2007;116:e418-e500; originally published online September 27, 2007; doi: 10.1161/CIRCULATIONAHA.107.185699. Pederson TR, et. al. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). 1994. Atheroscler Suppl. 2004 Oct;5(3):81-7. Steinberg D. Thematic review series: the pathogenesis of atherosclerosis. An interpretive history of the cholesterol controversy, part V: the discovery of the statins and the end of the controversy. J Lipid Res. 2006 Jul;47(7):1339-51. Epub 2006 Apr 3. http://www.cardiovascularbusiness.com/topics/healthcare-economics/slides-generics-push-statin-revenues-down-7b http://www.uptodate.com.libproxy.unm.edu/contents/simvastatin-drug-information detectedLanguage=en&source=search_result&search=simvastatin&selectedTitle=1~144&provider=noProvider#F221287 Berensen, A.(2006, June 23). Merck loses protection for patent on Zocor. The New York Times. http://www.nytimes.com/2006/06/23/business/23statin.html http://ymcacnm.org/join/membership-rates/ Pollack A. (2013, May 30). A Lone Voice Raises Alarms on Lucrative Diabetes Drugs. The New York Times. http://www.nytimes.com/2013/05/31/business/a-doctor-raises-questions-about-a-diabetes-drug.html?pagewanted=all&_r=0 Matveyenko AV, et. al. Beneficial Endocrine but Adverse Exocrine Effects of Sitagliptin in the Human Islet Amyloid Polypeptide Transgenic Rat Model of Type 2 Diabetes: Interactions With Metformin. Diabetes 58:1604–1615, 2009 Elashoff M, et. al. Pancreatitis, Pancreatic, and Thyroid Cancer With Glucagon-Like Peptide-1–Based Therapies. Gastroenterology, Volume 141, Issue 1, July 2011, Pages 150–156. http://dx.doi.org.libproxy.unm.edu/10.1053/j.gastro.2011.02.018 Butler AE, et. al. Marked Expansion of Exocrine and Endocrine Pancreas With Incretin Therapy in Humans With Increased Exocrine Pancreas Dysplasia and the Potential for Glucagon-Producing Neuroendocrine Tumors. Diabetes July 2013 vol. 62 no. 7 2595-2604. Gier B. Chronic GLP-1 Receptor Activation by Exendin-4 Induces Expansion of Pancreatic Duct Glands in Rats and Accelerates Formation of Dysplastic Lesions and Chronic Pancreatitis in the KrasG12D Mouse Model. Diabetes May 2012 vol. 61 no. 5 1250-1262. http://www.uptodate.com.libproxy.unm.edu/contents/glucagon-like-peptide-1-based-therapies-for-the-treatment-of-type-2-diabetesmellitus?detectedLanguage=en&source=search_result&search=sitagliptin&selectedTitle=5~23&provider=noProvider#H13 Engel SS, et. al. Safety and Tolerability of Sitagliptin in Type 2 Diabetes: Pooled Analysis of 25 Clinical Studies. Diabetes Therapy. June 2013, Volume 4, Issue 1, pp 119-145.