Acid-Base Physiology
advertisement

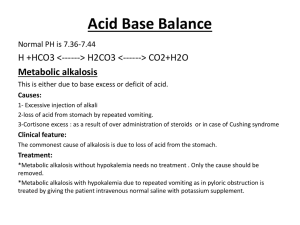
Acid-Base Physiology 2012 Objectives Understand normal mechanisms and regulation of acid-base balance Interpret blood gases Understand the effects of acidosis and alkalosis Evaluate and manage acidosis and alkalosis Normal Physiology Acid-base balance maintained by normal pulmonary excretion of CO2 and renal excretion of acid Organic buffers: HCO3-, HPO4, protein anions, carbonate 90% of bicarb is reabsorbed by kidney Renal excretion – H+ combines with urinary titratable acids (phosphates) or ammonia to form ammonium Henderson-Hasselbach equation: pH = 6.1 + log (HCO3 ÷ (0.03 x PCO2)) Compensatory Mechanisms pH is determined by ratio of HCO3 and PCO2 Body responds to changes in pH by attempting to normalize the pH Buffering Respiratory – alterations in paCO2 Renal – alterations in bicarbonate excretion Compensatory Mechanisms Compensated metabolic acidosis: Compensated metabolic alkalosis: 0.7 mmHg in pCO2 for every 1 meq/L in HCO3 Compensated respiratory acidosis: 1.2 mmHg in pCO2 for every 1 meq/L in HCO3 Acute- 1 meq/L for every 10 mmHg in pCO2 Chronic- 3.5 meq/L for every 10 mmHg in pCO2 Compensated respiratory alkalosis: Acute- 2 meq/L for every 10 mmHg in pCO2 Chronic- 4 meq/L for every 10 mmHg in pCO2 Blood Gas Interpretation General guidelines: 1) Is it acidosis or alkalosis? Acidosis – pH < 7.38 Alkalosis – pH > 7.42 2) Is it primary respiratory or metabolic? Evaluate paCO2 and bicarbonate 3) Is there compensation? Calculations from previous slides Blood Gas Interpretation 4) If respiratory disturbance, is it acute or chronic? Respiratory acidosis: Acute decrease in pH = 0.08 x (paCO2-40)/10 Chronic decrease in pH = 0.03 x (paCO2-40)/10 Respiratory alkalosis Acute increase in pH = 0.08 x (40-paCO2)/10 Chronic increase in pH = 0.03 x (40-paCO2)/10 5) If metabolic disturbance, is there an anion gap? Check serum Na, Cl, HCO3 Case #1 pH 7.16, pCO2 70, HCO3 24 Acidosis or alkalosis? Respiratory or metabolic? Compensated? Clinical Scenario? Case #1 pH 7.16, pCO2 70, HCO3 24 Acidosis or alkalosis? Respiratory or metabolic? Compensated? Clinical Scenario? Case #1 pH 7.16, pCO2 70, HCO3 24 Acidosis or alkalosis? Respiratory or metabolic? Compensated? Clinical Scenario? Case #1 pH 7.16, pCO2 70, HCO3 24 Acidosis or alkalosis? Respiratory or metabolic? Compensated? No … likely acute Clinical Scenario? Case #1 pH 7.16, pCO2 70, HCO3 24 Acidosis or alkalosis? Respiratory or metabolic? Compensated? Clinical Scenario? 2 yo receiving deep sedation by the adult ED attending who gives him 4 mg morphine, respiratory rate is 6 Acute Respiratory Acidosis Respiratory pathophysiology – airway obstruction, severe pneumonia, chest trauma, pneumothorax Acute drug intoxication (narcotics, sedatives) Residual neuromuscular blockade CNS disease (head trauma, decreased consciousness) Bicarbonate is often normal, kidneys have not had time to compensate Case #2 pH 7.6, pCO2 23, HCO3 22 Acidosis or alkalosis? Respiratory or metabolic? Compensated? Clinical scenario? Case #2 pH 7.6, pCO2 23, HCO3 22 Acidosis or alkalosis? Respiratory or metabolic? Compensated? Clinical scenario? Case #2 pH 7.6, pCO2 23, HCO3 22 Acidosis or alkalosis? Respiratory or metabolic? Compensated? Clinical scenario? Case #2 pH 7.6, pCO2 23, HCO3 22 Acidosis or alkalosis? Respiratory or metabolic? Compensated? No … likely acute Clinical scenario? Case #2 pH 7.6, pCO2 23, HCO3 22 Acidosis or alkalosis? Respiratory or metabolic? Compensated? Clinical scenario? 4 mo mechanically ventilated pt who was bagged during transport to CT scan by an overeager intern Respiratory Alkalosis Pain, anxiety Hypoxemia Interstitial lung disease Severe congestive heart failure (pulmonary edema) Pulmonary emboli Drugs – salicylates, methylxanthines, nicotine Sepsis, fever Hepatic failure – encephalopathy Pregnancy Thyrotoxicosis CNS hemorrhage Overagressive mechanical ventilation Case #3 pH 7.29, pCO2 26, HCO3 12 Acidosis or alkalosis? Respiratory or metabolic? Compensated? Clinical scenario? Case #3 pH 7.29, pCO2 26, HCO3 12 Acidosis or alkalosis? Respiratory or metabolic? Compensated? Clinical scenario? Case #3 pH 7.29, pCO2 26, HCO3 12 Acidosis or alkalosis? Respiratory or metabolic? Compensated? Clinical scenario? Compensatory Mechanisms Compensated metabolic acidosis: 1.2 mmHg in pCO2 for every 1 meq/L in HCO3 Compensated metabolic alkalosis: 0.7 mmHg in pCO2 for every 1 meq/L in HCO3 Compensated respiratory acidosis: Acute- 1 meq/L for every 10 mmHg in pCO2 Chronic- 3.5 meq/L for every 10 mmHg in pCO2 Compensated respiratory alkalosis: Acute- 2 meq/L for every 10 mmHg in pCO2 Chronic- 4 meq/L for every 10 mmHg in pCO2 Case #3 pH 7.29, pCO2 26, HCO3 12 Acidosis or alkalosis? Respiratory or metabolic? Compensated? Yes … 1.2 mmHg decrease in pCO2 for every 1 meq/L decrease in HCO3 Clinical scenario? Case #3 pH 7.29, pCO2 26, HCO3 12 Acidosis or alkalosis? Respiratory or metabolic? Compensated? Clinical scenario? 10 yo dev delayed pt admitted with diarrhea, med list reveals mom has been giving Miralax every 4 hours Metabolic Acidosis Anion gap Metabolic Acidosis Anion gap Lactic acidosis DKA Toxic ingestions (salicylates, ethylene glycol, ethanol, isopropyl alcohol, paraldehyde, methanol) Renal failure – uremia Metabolic Acidosis Nonanion gap Metabolic Acidosis Nonanion gap RTA Diarrhea Hypoaldosteronism Potassium-sparing diuretics Pancreatic loss of bicarbonate Ureteral diversion Carbonic anhydrase inhibitors Acid administration (ArgCl, NaCl) Case #4 pH 7.47, pCO2 46, HCO3 32 Acidosis or alkalosis? Respiratory or metabolic? Compensated? Clinical scenario? Case #4 pH 7.47, pCO2 46, HCO3 32 Acidosis or alkalosis? Respiratory or metabolic? Compensated? Clinical scenario? Case #4 pH 7.47, pCO2 46, HCO3 32 Acidosis or alkalosis? Respiratory or metabolic? Compensated? Clinical scenario? Compensatory Mechanisms Compensated metabolic acidosis: 1.2 mmHg in pCO2 for every 1 meq/L in HCO3 Compensated metabolic alkalosis: 0.7 mmHg in pCO2 for every 1 meq/L in HCO3 Compensated respiratory acidosis: Acute- 1 meq/L for every 10 mmHg in pCO2 Chronic- 3.5 meq/L for every 10 mmHg in pCO2 Compensated respiratory alkalosis: Acute- 2 meq/L for every 10 mmHg in pCO2 Chronic- 4 meq/L for every 10 mmHg in pCO2 Case #4 pH 7.47, pCO2 46, HCO3 32 Acidosis or alkalosis? Respiratory or metabolic? Compensated? Yes … 0.7 mmHg increase in pCO2 for every 1 meq/L increase in HCO3 Clinical scenario? Case #4 pH 7.47, pCO2 46, HCO3 32 Acidosis or alkalosis? Respiratory or metabolic? Compensated? Clinical scenario? 5 yo s/p appendectomy with NG tube left to suction on 7CH for 5 days Metabolic Alkalosis Chloride-responsive (urine Cl < 10 meq/L) Gastric acid loss (vomiting, NG suction) Contraction alkalosis (often due to loop or thiazide diuretics) Posthypercapnia syndrome Metabolic Alkalosis Chloride-resistant Mineralocorticoid excess Renal chloride wasting (Bartter syndrome) Exogenous alkali (milk-alkali syndrome, massive blood transfusion) Hypokalemia Case #5 pH 7.30, pCO2 89, HCO3 42 Acidosis or alkalosis? Respiratory or metabolic? Compensated? Clincial scenario? Case #5 pH 7.30, pCO2 89, HCO3 42 Acidosis or alkalosis? Respiratory or metabolic? Compensated? Clincial scenario? Case #5 pH 7.30, pCO2 89, HCO3 42 Acidosis or alkalosis? Respiratory or metabolic? Compensated? Clincial scenario? Compensatory Mechanisms Compensated metabolic acidosis: 1.2 mmHg in pCO2 for every 1 meq/L in HCO3 Compensated metabolic alkalosis: 0.7 mmHg in pCO2 for every 1 meq/L in HCO3 Compensated respiratory acidosis: Acute- 1 meq/L for every 10 mmHg in pCO2 Chronic- 3.5 meq/L for every 10 mmHg in pCO2 Compensated respiratory alkalosis: Acute- 2 meq/L for every 10 mmHg in pCO2 Chronic- 4 meq/L for every 10 mmHg in pCO2 Case #5 pH 7.30, pCO2 89, HCO3 42 Acidosis or alkalosis? Respiratory or metabolic? Compensated? Yes … 3.5 meq/L increase in HCO3 for every 10 mmHg increase in CO2 Clincial scenario? Case #5 pH 7.30, pCO2 89, HCO3 42 Acidosis or alkalosis? Respiratory or metabolic? Compensated? Clincial scenario? 35 yo CF patient on the Peds floor with end-stage lung disease Chronic Respiratory Acidosis Chronic lung diseases (BPD, CF) Neuromuscular disorders Severe restrictive lung disease Severe obesity Case #6 pH 6.84, pCO2 82, HCO3 14 Acidosis or alkalosis? Respiratory or metabolic? Compensated? Clinical scenario? Case #6 pH 6.84, pCO2 82, HCO3 14 Acidosis or alkalosis? Respiratory or metabolic? Compensated? Clinical scenario? Blood Gas Interpretation 4) If respiratory acidosis or alkalosis, is it acute or chronic? Respiratory acidosis: Acute decrease in pH = 0.08 x (paCO2-40)/10 Chronic decrease in pH = 0.03 x (paCO2-40)/10 Respiratory alkalosis Acute increase in pH = 0.08 x (40-paCO2)/10 Chronic increase in pH = 0.03 x (40-paCO2)/10 Case #6 pH 6.84, pCO2 82, HCO3 14 Acidosis or alkalosis? Respiratory or metabolic? Compensated? Clinical scenario? Case #6 pH 6.84, pCO2 82, HCO3 14 Acidosis or alkalosis? Respiratory or metabolic? Compensated? No … Combined acidosis Clinical scenario? Case #6 pH 6.84, pCO2 82, HCO3 14 Acidosis or alkalosis? Respiratory or metabolic? Compensated? Clinical scenario? 2 mo found down at home, hypoperfusion leading to lactic acidosis, hypoventilation leading to respiratory acidosis Case # 7 pH 7.46, pCO2 24, HCO3 16 Acidosis or alkalosis? Respiratory or metabolic? Compensated? Clinical scenario? Case # 7 pH 7.46, pCO2 24, HCO3 16 Acidosis or alkalosis? Respiratory or metabolic? Compensated? Clinical scenario? Case # 7 pH 7.46, pCO2 24, HCO3 16 Acidosis or alkalosis? Respiratory or metabolic? Compensated? Clinical scenario? Case # 7 pH 7.46, pCO2 24, HCO3 16 Acidosis or alkalosis? Respiratory or metabolic? Compensated? Well … 2 meq/L decrease in HCO3 for every 10 mmHg decrease in CO2 Combined respiratory alkalosis & metabolic acidosis Clinical scenario? Case # 7 pH 7.46, pCO2 24, HCO3 16 Acidosis or alkalosis? Respiratory or metabolic? Compensated? Clinical scenario? Anxious 5 yo who is hyperventilating and has a history of RTA Case #8 pH 7.45, pCO2 54, HCO3 36 Acidosis or alkalosis? Respiratory or metabolic? Compensated? Clinical scenario? Case #8 pH 7.45, pCO2 54, HCO3 36 Acidosis or alkalosis? Respiratory or metabolic? Compensated? Clinical scenario? Case #8 pH 7.45, pCO2 54, HCO3 36 Acidosis or alkalosis? Respiratory or metabolic? Compensated? Clinical scenario? Case #8 pH 7.45, pCO2 54, HCO3 36 Acidosis or alkalosis? Respiratory or metabolic? Compensated? No … 0.7 mmHg increase for every 1 meq/L predicts pCO2 of 48 Combined metabolic alkalosis and respiratory acidosis Clinical scenario? Case #8 pH 7.45, pCO2 54, HCO3 36 Acidosis or alkalosis? Respiratory or metabolic? Compensated? Clinical scenario? 1 yo with vomiting for 3 days who presents to the ED with lethargy and decreased arousal, hypoventilating Physiologic Effects of Acidosis Shifts the oxygen-hemoglobin dissociation curve to the right Decreased affinity for O2 Physiologic Effects of Acidosis Pulmonary effects – vasoconstriction decreases pulmonary blood flow Cardiac effects – depressed contractility Neurologic effects – increased cerebral blood flow, increased ICP Extracellular shift of K+ hyperkalemia Sympathetic overactivity, resistance to catecholamines Physiologic Effects of Alkalosis Shifts the oxygen-hemoglobin dissociation curve to the left Stronger bond between Hb and O2 Decreased O2 delivery to tissues Physiologic Effects of Alkalosis Cardiac arrhythmias Lungs – vasodilation increases pulmonary blood flow Neurologic effects – headache, seizures, altered mental status Decreased cerebral blood flow from vasoconstriction Decreased levels of ionized Ca++ Intracellular shift of potassium severe hypokalemia Management - Respiratory Acidosis Treat the underlying disorder Assist or increase ventilation Secure airway if necessary Increase tidal volume or respiratory rate if mechanically ventilated Noninvasive ventilation Bronchodilators Reverse sedative medications Management – Respiratory Alkalosis Treat the underlying disorder Decrease ventilation Decrease respiratory rate Decrease tidal volume Sedation and pain control Reassurance for anxious patients Management - Metabolic Disorders Acidosis Treat the underlying disorder Consider bicarb administration depending on etiology Dialysis in the setting of renal failure Alkalosis Treat the underlying disorder Chloride-responsive: replete chloride (NaCl, KCl, ArgCl) Carbonic-anydrase inhibitors if diuresis needed Conclusion The body has compensatory mechanisms to maintain acid-base balance. Blood gases should be interpreted in a systematic way to determine the etiology of the acid-base disturbance. Acidosis causes pulmonary vasoconstriction, cardiac depression, arterial vasodilation, & decreased O2 affinity. Alkalosis causes pulmonary vasodilation, arterial vasoconstriction, & increased O2 affinity. Management of acid-base disorders primarily involves treatment of the underlying disorder. Everyone always has slides of their kids … QUESTIONS? References www.uptodate.com www.emedicine.com Morganroth ML. Six steps to acid-base analysis: clinical applications. The Journal of Critical Illness. 1990;5:460-69. Morganroth ML. An analytic approach to diagnosing acidbase disorders. The Journal of Critical Illness. 1990;5:138-50.