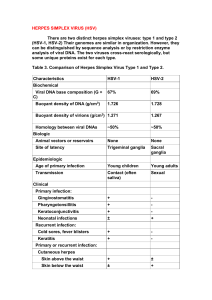
HSV-1 and HSV-2:
Chemotherapy
Review: Chemotherapeutic Agents to
Treat Viral Infections
• Control of Viruses Since viruses lack
the structures and metabolic processes
that are altered by common antibiotics,
antibiotics are virtually useless in
treating viral infections.To date, only a
few chemotherapeutic agents have
been found to be somewhat effective
against just a few limited viruses.
Antiviral Drugs (general)
• 1. amantadine (Symmetrel): used
prophylactically against influenza A in
high-risk individuals.
• 2. rimantidine (Flumadine): used for
treatment and prophylaxis of influenza
A.
Antiviral Drugs (general)
• 3. zanamivir (Relenza): used to limit
the duration of influenza A and B
infections.
• 4. oseltamivir (Tamiflu): used limit the
duration of influenza infections.
OH
OH
OH
O
COOH
O
O
COO Et
O
N
H
HN
NH
NH2
Z anam ivir (R elenza)
NH
NH2
O seltam ivir (T am iflu)
What is HSV?
• HSV = Herpes Simplex Virus
• HSV-1 is the ‘usual’ cause of cold sores
• HSV-2 is the ‘usual’ cause of genital
herpes
• Both types look the same under the
microscope and share about 50% of
their DNA.
http://www.healthscout.com/an
imation/68/21/main.html
What is the difference
between HSV-1 and HSV-2?
• Both types infect the body’s mucosal
surfaces, usually mouth or genitals, and then
establish latency in the nervous system.
• For both types, at least two-thirds of the
infected people have no symptoms, or
symptoms too mild to notice.
• However, both types can recur and spread,
even after a period in which there were no
symptoms.
The differences
• HSV-1 usually establishes latency in the
trigeminal ganglion, a collection of nerve
cells near the ear. Then it tends to
recur on the lower lip or face.
• HSV-2 usually establishes latency in
sacral ganglion at the base of the spine.
From there, it recurs in the genital area.
HSV-1
• However, one can have HSV-1 both genitally
and orally.
• HSV-1 is usually mild, especially when it
infects the lips, face, or genitals.
• However, HSV-1 can recur in the eye,
causing ocular herpes, which can lead to
blindness, and can even spread
spontaneously to the brain, causing herpes
encephalitis, which can lead to death.
HSV-2
• 22% of adult Americans have HSV-2
• Like HSV-1, HSV-2 symptoms are usually
mild, so mild, in fact, that two-thirds of
infected people don’t know they have it.
• HSV-2 rarely causes complications or
spreads to other parts of the body.
• Oral HSV-2 infections are rare. But even
when an infection does occur, recurrent oral
outbreaks are uncommon.
Transmission of HSV-2
• In the first year of HSV-2 infection, people
shed the virus from the genital area about 6
to 10% of those days when they are
asymptomatic. This decreases over time and
can also be further lessened by the use of
oral medication. Sex should be avoided in
the presence of symptomatic lesions.
• Having a previous HSV-1 infection seems to
provide some immunity to an HSV-2 infection.
This is probably the reason that oral HSV-2
infections are rare, given the studies which
show that a significant proportion of the
population practices oral sex.
How severe an infection?
• HSV is a lifelong illness
• But HSV-2 usually produces only mild
symptoms or signs or no symptoms at all.
However, HSV-2 can cause recurrent painful
genital sores in many adults, and HSV-2
infection can be severe in people with
suppressed immune systems.
• Another factor is how long a person has had
the infection. It seems to decrease in severity
over time, for reasons which are unclear.
Symptoms
• If signs and symptoms occur during the
first episode, they can be quite
pronounced. The first episode usually
occurs within two weeks after the virus
is transmitted, and the sores typically
heal within two to four weeks.
• Other signs and symptoms during the
primary episode may include a second
crop of sores, or flu-like symptoms,
including fever and swollen glands.
Is there a cure?
• There is no treatment that can
cure herpes, but antiviral
medications can shorten and
prevent outbreaks during the
period of time the person takes the
medication.
Vaccines?
• NIH is now in the midst of Phase III
clinical trial of an HSV-2 vaccine. This
vaccine appears to be about 50%
effective.
• If approved, it would be available in
2008.
Antiviral Chemotherapy for HSV
• There are several prescription antiviral
medications for controlling herpes outbreaks,
include acyclovir (Zovirax), valacyclovir
(Valtrex), famcyclovir (Famvir), and
pencyclovir.
• Acyclovir was the original and prototypical
member of this class
• Valacyclovir and famcyclovir are prodrugs of
acyclovir and pencyclovir respectively, with
improved oral bioavailability.
Mechanism of Action of
Antivirals to treat HSV
• Both acyclovir and pencyclovir work by
interfering with viral replication, effectively
slowing the replication rate of the virus, and
providing a greater opportunity for the
immune response to intervene.
• All drugs in this class depend on the activity
of the viral thymidine kinase to convert the
drug to a monophosphate form and
subsequently interfere with viral DNA
replication.
DNA Virus
• Recall that HSV is a DNA virus (influenza was
an RNA virus)
• In general, more drugs are available to treat
DNA viruses than for RNA viruses (excluding
those used to treat HIV).
• Most of the drugs available for treatment of
DNA viruses have been developed against
herpesviruses.
• Diseases include cold sores, genital herpes,
chickenpox, shingles, mononucleosis, etc.
Acyclovir
• Discovered by random compound
screening and introduced into the
market in 1981.
• It was the first non-toxic herpes drug to
be used systemically.
• It is used for the treatment of infections
due to both HSV-1 and HSV-2.
http://www.cat.cc.md.us/biotut
orials/dna/dnareppr.html
• http://www.dnalc.org/ddnalc/resources/s
angerseq.html
• Aciclovir interferes with DNA synthesis, but must first become activated.
•To become activated, Aciclovir must be phosphorylated (3x)
• However, Aciclovir itself is not a good substrate for mammalian kinases,
thus it relies on the viral thymidine kinase to become phosphorylated the
first time.
• This is good, since the drug cannot interfere with DNA synthesis in cells
that are not infected with the virus, thus reducing the toxicity of the drug.
• The second and third phosphorylations, however, are performed by the
cellular thymidylate kinase.
•Aciclovir triphosphate is mistaken for
deoxyguanosine triphosphate.
• However, since it lacks the 3’-OH
group, it cannot be linked to the
adjacent residue in the ‘usual’
fashion.
Viral Resistance to Aciclovir
• Aciclovir-resistant strains of herpes are
appearing.
• This occurs when mutations of the viral
thymidine kinase result in an enzyme which
no longer phosphorylates aciclovir.
• Or when the viral DNA polymerase mutates to
a form that no longer recognizes the activated
drug.
Prodrugs of Aciclovir
• Aciclovir itself is polar, and thus the oral
bioavailability is low.
• A valine ester of aciclovir, known as valaciclovir, is
more bioavailable. This ester is cleaved by
esterases.
• Also, the C6 oxygen can be removed to produce
desciclovir, also a prodrug, which is converted to
aciclovir by xanthine oxidase.
Families of herpesviruses
• Aciclovir is effective against the a-subfamily of
herpesviruses, but not against the b-subfamily.
• However, other drugs, such as ganciclovir, which
has an additional hydroxyl group, thus resembling
the natural substrate a bit more closely.
• Ganciclovir is phosphorylated by thymidine kinases produced by both
the a- and the b-subfamilies of herpes viruses.
• Thus ganciclovir can be used to treat cytomegalovirus (CMV) infections
•Cytomegalovirus is a common virus that infects most people worldwide.
CMV infection is usually harmless and a healthy immune system can
hold the virus in check. However, if a person's immune system is
seriously weakened, the virus can become active and cause CMV
disease.
•A less polar analog is valganciclovir, which is a valine ester at one of the
hydroxyl groups.
• Other modifications include substituting the ether oxygen
with a methylene (CH2) to produce penciclovir and
famciclovir (diacetate ester of penciclovir)
• These drugs are used topically for the treatment of cold
sores and intravenously for the treatment of HSV in
immunocompromised indivuals.
• Some viruses are immune from the action of this class
of antiviral agents, due to their lack of the thymidine
kinase enzyme.
• The agent cidofovir (shown above) was designed to
overcome this problem by incorporating an appropriately
placed phosphonomethylene group to mimic the
phosphate of deoxycytidine monophosphate.
• However, with the added phosphono group, the drug is
extremely polar and has low oral bioavailability.
• The three nucleoside analogs shown above are
mistaken for the structurally related nucleosides.
• These compounds appear to inhibit viral DNA
polymerase.
• Foscarnet is used to treat ganciclovir-resistant CMV or
to treat aciclovir-resistant HSV. It has renal toxicity.
• Foscarnet it a non-competitive inhibitor of viral DNA
polymerase.
HIV
• HIV = Human Immuno-deficiency Virus
• HIV is an RNA virus which contains two identical
strands of (+)ssRNA in its capsid.
• HIV is a retrovirus (i.e. viral RNS serves as template
for the synthesis of a complementary DNA)
• HIV infection usually progresses to AIDS
• AIDS = Acquired Immunodeficiency Syndrome.
• This stage of HIV infection is usually characterized by
opportunistic diseases, including Pneumocystis
carinii pneumonia, Kaposi sarcoma, cytomegalovirus
disease, etc.
HIV Introduction
• HIV-1 is responsible for AIDS in
America, Europe, and Asia
• HIV-2 occurs mainly in western Africa
• At present, anti-HIV drugs are aimed at
two targets: reverse transcriptase and
HIV protease.
• Good animation of HIV-1 Lifecycle:
• http://www.hopkinsaids.edu/hiv_lifecycle/hivcycle_txt.html
Antiretroviral Agents Currently Available (generic
name/Trade name) Nucleoside Analogs
•
•
•
•
•
•
zidovudine/Retrovir(AZT, ZDV)
didanosine/Videx, Videx EC (ddI)
zalcitabine/HIVID (ddC)
stavudine/Zerit (d4T)
lamivudine/Epivir (3TC)
abacavir/Ziagen (ABC)
Animation of action of AZT
• http://www.uri.edu/pharmacy/animation/
dnaHanleyAnim.htm
• http://www.cat.cc.md.us/courses/bio141/
lecguide/unit2/viruses/vircontrol.html
Non-Nucleoside Reverse Transcriptase
Inhibitors (NNRTI’s)
• nevirapine/Viramune (NVP)
• delavirdine/Rescriptor (DLV)
• efavirenz/Sustiva (EFV)
•NNRTI’s are generally hydrophobic molecules that
bind to an allosteric binding site
•Binding to this allosteric site locks the neighboring
substrate-binding site into an inactive conformation.
•However, resistance to NNRTI’s can develop rapidly,
and thus they are used in combination with NRTI’s
Protease Inhibitors
•
•
•
•
•
•
indinavir/Crixivan
ritonavir/Norvirs
aquinavir/Invirase, Fortovase
nelfinavir/Viracept
amprenavir/Ageneras
elopinavir/ritonavir, Kaletra
Nucleoside Reverse Transcriptase Inhibitors (NRTI’s)
Non-nucleoside reverse
transcriptase inhibitors
Chemical Mechanism of HIV
Protease Hydrolysis
Modeling an inhibitor after the
transition state may result in a
tighter-binding inhibitor
But the actual transition state (in box above) is chemically
unstable, so a number of more stable “transition state
isosteres” have been devised.
Development of saquinavir