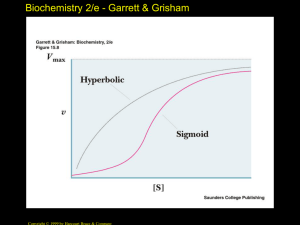
Biochemistry 2/e - Garrett & Grisham
CHAPTER 1
Chemistry is the Logic of
Biological Phenomena
to accompany
Biochemistry, 2/e
by
Reginald Garrett and Charles Grisham
All rights reserved. Requests for permission to make copies of any part of the work
should be mailed to: Permissions Department, Harcourt Brace & Company,
6277 Sea Harbor Drive, Orlando, Florida 32887-6777
Biochemistry 2/e - Garrett & Grisham
Outline
• 1.1 Distinctive Properties of Living
Systems
• 1.2 Biomolecules: Molecules of Life
• 1.3 Biomolecular Hierarchy
• 1.4 Properties of Biomolecules
• 1.5 Organization and Structure of Cells
• 1.6 Viruses as Cell Parasites
Biochemistry 2/e - Garrett & Grisham
On Life and Chemistry...
• “Living things are composed of lifeless
molecules” (Albert Lehninger)
• “Chemistry is the logic of biological
phenomena” (Garrett and Grisham)
Biochemistry 2/e - Garrett & Grisham
1.1 Distinctive Properties of
Living Systems
• Organisms are complicated and highly
organized
• Biological structures serve functional
purposes
• Living systems are actively engaged in
energy transformations
• Living systems have a remarkable
capacity for self-replication
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
1.2 Biomolecules: The
Molecules of Life
H, O, C and N make up 99+% of atoms in the
human body
ELEMENT
Oxygen
Hydrogen
Carbon
Nitrogen
PERCENTAGE
63
25.2
9.5
1.4
Biochemistry 2/e - Garrett & Grisham
1.2 Biomolecules: The
Molecules of Life
• What property unites H, O, C and N and
renders these atoms so appropriate to
the chemistry of life?
• Answer: Their ability to form covalent
bonds by electron-pair sharing.
Biochemistry 2/e - Garrett & Grisham
1.2 Biomolecules: The
Molecules of Life
What are the bond energies of covalent
bonds?
Bond Energy kJ/mol
H-H
436
C-H
414
C-C
343
C-O
351
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
1.3 A Biomolecular Hierarchy
Simple Molecules are the Units for
Building Complex Structures
•
•
•
•
Metabolites and Macromolecules
Organelles
Membranes
The Unit of Life is the Cell
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
1.4 Properties of Biomolecules Reflect
Their Fitness to the Living Condition
• Macromolecules and Their Building
Blocks Have a “Sense” or Directionality
• Macromolecules are Informational
• Biomolecules Have Characteristic
Three-Dimensional Architecture
• Weak Forces Maintain Biological
Structure and Determine Biomolecular
Interactions
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
1.4 Properties of
Biomolecules Reflect Their
Fitness to the Living Condition
•
•
•
•
Important numbers!
van der Waals: 0.4-4.0 kJ/mole
Hydrogen bonds: 12-30 kJ/mole
Ionic bonds: 20 kJ/mole
Hydrophobic interactions: <40 kJ/mole
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
Two Important Points About
Weak Forces
• Biomolecular Recognition is Mediated
by Weak Chemical Forces
• Weak Forces Restrict Organisms to a
Narrow Range of Environmental
Conditions
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
Organization and Structure of
Cells
• Prokaryotic cells
– A single (plasma) membrane
– no nucleus or organelles
• Eukaryotic cells
– much larger in size than prokaryotes
– 103-104 times larger!
– Nucleus plus many organelles
– ER, Golgi, mitochondria, etc.
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham
Biochemistry 2/e - Garrett & Grisham