Electrostatics
advertisement

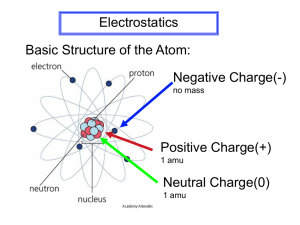
Electrostatics GIRL SAFELY CHARGED TO SEVERAL HUNDRED THOUSAND VOLTS GIRL IN GREAT DANGER AT SEVERAL THOUSAND VOLTS The Nature of Electric Charge Discovery of charge The Greeks first noticed electric charged by rubbing amber with fur, then picking up bits of matter. The Greek word for amber is elektron. Benjamin Franklin arbitrarily called the two kinds of charge positive and negative. In most cases, only the negative charge is mobile. Properties of charge Like charges repel, and unlike charges attract. Charge is conserved, meaning it cannot be created or destroyed, only transferred from one location to another. In all atoms, electrons (qe) have negative charge and protons (qp) have positive charge. Charge is quantized, meaning it comes in discrete amounts (like money). total charge = integer x fundamental unit of charge click for animation Insulators and Conductors Insulators In insulators, electrons are bound in “orbit” to the nucleus in each atom. When charge is placed on an insulator, it stays in one region and does not distribute. Wood, plastic, glass, air, and cloth are good insulators. Conductors In conductors electrons can move from atom to atom, thus electricity can “flow”. CHARGED INSULATOR When charge is placed on a conductor, it redistributes to the outer surface. Metals (copper, gold, and aluminum) are good conductors. CHARGED CONDUCTOR Polarization Polarization is the separation of charge In a conductor, “free” electrons can move around the surface of the material, leaving one side positive and the other side negative. In an insulator, the electrons “realign” themselves within the atom (or molecule), leaving one side of the atom positive and the other side of the atom negative. Polarization is not necessarily a charge imbalance! Charging by Friction POSITIVE Rabbit's fur Glass Mica Nylon Wool Cat's fur Silk Paper Cotton Wood Lucite Wax Amber Polystyrene Polyethylene Rubber ballon Sulfur Celluloid Hard Rubber Vinylite Saran Wrap NEGATIVE When insulators are rubbed together, one gives up electrons and becomes positively charged, while the other gains electrons and becomes negatively charged. Materials have different affinities for electrons. A triboelectric series rates this relative affinity. A material will give up electrons to another material below it on a triboelectric series. Common examples of charging by friction: • small shocks from a doorknob after walking on carpet with rubber-soled shoes • plastic foodwrap that sticks to a container • sweater pulled over your head that sparks • laundry from the dryer that clings • balloon rubbed with hair sticks that to a wall click for applet Charging by Conduction When a charged conductor makes contact with a neutral conductor there is a transfer of charge. CHARGING NEGATIVELY CHARGING POSITIVELY Electrons are transferred from the rod to the ball, leaving them both negatively charged. Electrons are transferred from the ball to the rod, leaving them both positively charged. Remember, only electrons are free to move in solids. Notice that the original charged object loses some charge. click for animation Charging by Induction Induction uses the influence of one charged object to “coerce” charge flow. Step 1. A charged rod is brought near an isolated conductor. The influence of the charge object polarizes the conductor but does not yet charge it. Step 2. The conductor is grounded to the Earth, allowing charge to flow out between it and the Earth. Charging by Induction (cont.) Step 3. The ground is removed while the charge rod is still nearby the conductor. Step 4. The rod is removed and the conductor is now charge (opposite of rod). An object charged by induction has the opposite sign of the influencing body. Notice that the original charged object does not lose charge. click for animation click for animation click for animation Electric Forces and Electric Fields CHARLES COULOMB (1736-1806) MICHAEL FARADAY (1791-1867) Electrostatic Charges A New Fundamental Physics Quantity Electrostatic charge is a fundamental quantity like length, mass, and time. The symbol for charge is q. The SI unit for charge is called the coulomb (C). ATTRACTION AND REPULSION The charge of an electron (qe) is -1.6 x 10-19 C The charge of an proton (qp) is 1.6 x 10-19 C Common electrostatic charges are small: millicoulomb = mC = 10-3 C microcoulomb = C = 10-6 C nanocoulomb = nC = 10-9 C The Electrostatic Force Charles Coulomb’s Torsion Balance A torsion balance measures the force between small charges. The electrostatic force depends directly on the magnitude of the charges. The force depends inversely on the square of distance between charges (another “inverse square law”)! COULOMB’S LAW OF ELECTROSTATIC FORCE constant charges kq1q2 Fe 2 r electrostatic force distance TORSION BALANCE The constant of proportionality, k, is equal to 9.0 x 109 Nm2/C2. A negative force is attractive, and a positive force is repulsive. The sign (+ or –) is different from a vector direction (left or right) The Electrostatic Force EXAMPLE 1 - Find the force between these two charges 9.0 10 5 10 9 Fe 6 C 8 10 6 C 0.04 m 2 Fe 225 N The negative signs means force of attraction, but does not indicate left or right direction EXAMPLE 2 - Find the net force on the left charge 9.0 10 5 10 9 Fe Fe 360 N 6 C 5 10 6 C 0.025 m 2 (force of repulsion) Fnet Fleft Fright Fnet 360 N 225 N 135 N, to the left The Electrostatic Force EXAMPLE 3 - Find the net force on the upper left charge Fe,x 225 N, right Fe,y 360 N, up Fe Fe,x 2 Fe,y 2 225 2 360 2 425 N Fe,y 1 360 tan tan 58.0Þ 225 Fe,x 1 425 N, 58˚ EXAMPLE 4 (Honors only) - Find the net force on the lower left charge 9.0 10 5 10 C–8 10 C 162 9 Fe1 -6 -6 0.04 0.025 m 2 2 Fe1,x 162 cos 32Þ 137.2 N 2.5 tan -1 32Þ 4 Fe1,y 162 sin 32Þ 85.75 N Fe Fe,x 2 Fe,y 2 137.2 2 (85.75 360)2 307 N 307 N, -63.4˚ Fe,y 1 85.75 360 tan 63.4Þ 137.2 Fe,x tan 1 Electric Field Strength Field Theory Visualizes Force At A Distance DEFINITION OF GRAVITATIONAL FIELD DEFINITION OF ELECTRIC FIELD force g field mass g E field force charge Fe E q0 Fg m q0 is a small, positive test charge Electric field is a vector quantity E field points toward negative charges E field points away from positive charges SI unit of electric field click for applet newton N coulomb C Electric Field Lines Single Point Charges POSITIVE CHARGE NEGATIVE CHARGE Density of field lines indicates electric field strength Inverse square law obeyed click for applet Definition of E Field for single point charge Fe kq0 q / r 2 E q0 q0 electric field constant charge kq E 2 r distance Electric Field Lines Electric fields for multiple point charges click for applet click for applet click for applet POSITIVE AND NEGATIVE POINT CHARGES TWO POSITIVE POINT CHARGES OPPOSITE MAGNETIC POLES ALIKE MAGNETIC POLES Electric Fields EXAMPLE 1 Find the electric field strength at 2 meters from the 5 millicoulomb charge. kq E 2 r E 9 10 E 9 Nm 2 /C2 5 10 3 C 2 m 2 E=1.13 107 N/C, to the right EXAMPLE 2 Find the force on an proton placed 2 meters from the 5 millicoulomb charge in the problem above. E Fe q 9 10 F e Fe qE 1.6 10-19 C 1.13 10 7 N/C 1.81 10-12 N, to the right 9 OR Nm 2 /C2 5 10 3 C 1.6 10-19 C 2 m 2 1.8 10 -12 N, to the right Electric Potential Energy Electric Potential Energy versus Gravitational Potential Energy FALLING MASS VS. FALLING CHARGE Electric potential energy (PE) is stored when a positive charge is moved against an electric field. Potential energy can be converted to kinetic energy, heat, light, sound etc. Potential energy is a scalar quantity measured in joules (J). STORING POTENTIAL ENERGY POTENTIAL ENERGY GAIN OR LOSS positive (+) charge negative (–) charge toward E loses PE gains PE opposite E gains PE loses PE PE for Constant Electric Field CONSTANT GRAVITATIONAL FIELD CONSTANT ELECTRIC FIELD PE mgh PE qEd Example electric potential energy charge E field How much potential energy is converted when an electron is accelerated through 0.25 m in a cathode ray tube (TV set) with an electric field strength of 2 x 105 N/C? PE qEd (1.6 1019 ) (2 105 )(0.25) 8.0 1015 J distance PE for Two Point Charges (Honors only) Potential Energy is force times distance kq1q2 PE Fe d 2 r r electric potential energy constant charges kq1q2 PE r distance Potential energy is positive for like charges Potential energy is negative for opposite charges Potential energy is zero at infinite distance Example How much electrostatic potential energy in a hydrogen atom, which consists of one electron at a distance of 5.3 x 10-11 meters from the nucleus (proton). kq1q2 (9 10 9 )(1.6 10 19 )(–1.6 10 19 ) 18 PE 4.35 10 J 11 r 5.3 10 Potential Difference (Voltage) Electric potential is average energy per charge. Energy Potential Charge Energy is a relative quantity (absolute energy doesn’t exist), so the change in electric potential, called potential difference, is meaningful. PE A good analogy: potential is to temperature, as V potential energy is to heat. q Potential difference is often called voltage. Voltage is only dangerous when a lot of energy is transferred. click for web page SI Units Voltage, like energy, is a scalar. A volt (v) is the unit for voltage named in honor of Alessandro Volta, inventor of the first battery. 1 joule J 1 volt V 1 coulomb C source voltage (V) common dry cell 1.5 car battery 12 household (US) 120 comb through hair 500 utility pole 4,400 transmission line 120,000 Van de Graaff 400,000 lightning 1,000,000,000 Potential Difference (Voltage) A SEVERAL THOUSAND VOLT POWERLINE CAN ILLUMINATE A FLUORESCENT LIGHT A PARACHTUE ACCIDENT LANDED THIS MAN ON A 138,000 THOUSAND VOLT LINE, BUT HE SUFFERED ONLY MINOR BURNS Potential Difference for Constant Electric Field Potential energy is often stored in a capacitor. Capacitors are made by putting an insulator in between two conductors. Most capacitors have constant electric fields. PE qEd V q q V Ed voltage E field distance Example Calculate the magnitude of the electric field set up in a 2-millimeter wide capacitor connected to a 9-volt battery. V Ed 9 E(0.002) E 4500 N/C Potential Difference for Point Charge (Honors only) Consider a test charge to measure potential PE kqq0 / r V q0 q0 constant potential difference charge kq V r distance Example -4 nC 0.3 m find ∆V here 0.4 m 10 nC 6 nC kq1 (9 10 9 )(6 10 9 ) V1 180 V r 0.3 kq2 (9 10 9 )(4 10 9 ) V2 90 V r 0.4 kq3 (9 10 9 )(10 10 9 ) V3 180 V r 0.5 V V1 V2 V3 180 90 180 270 V Summary of Electrostatic Equations Electrostatic Force kq1q2 Fe 2 r force between two charges Electric Field Fe E q0 definition kq E 2 r for point charge Potential Energy PE qEd for constant E field kq1q2 PE r Honors only! for two charges Potential Difference PE V q kq V r definition for point charge V Ed for constant E field