phys586-lec18

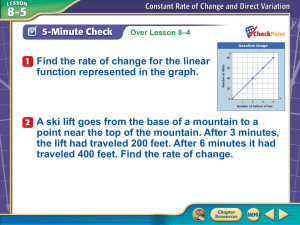
Ionization Detectors
Basic operation
Charged particle passes through a gas
(argon, air, …) and ionizes it
Electrons and ions are collected by the detector anode and cathode
Often there is secondary ionization producing amplification
1
Ionization Detectors
Modes of operation
Ionization mode
Full charge collection but no amplification (gain=1)
Generally used for gamma exposure and large fluxes
Proportional mode
Ionization avalanche produces an amplified signal proportional to the original ionization (gain = 10 3— 10 5 )
Allows measurement of dE/dx
Limited proportional (streamer) mode
Secondary avalanches from strong photo-emission and space charge effects occur (gain = 10 10 )
Geiger-Muller mode
Massive photo-emission results in many avalanches along the wire resulting in a saturated signal
2
Ionization Detectors
3
Ionization
Ionization
Direct – p + X -> p + X + + e -
Penning effect - Ne * + Ar -> Ne + Ar + + e -
n total
= n primary
+ n secondary
4
Ionization
The number of primary e/ion pairs is Poisson distributed, being due to a small number of independent interactions
1
P
1
e
n p rima ry n primary for 1mm Ar
2 .
5 gives
0 .
92
Total number of ions formed is n total
roughly, dE
x dx
, W i is the effective
W i n total
2
4
n primary ave.
energy to make an ion pair
5
Ionization air 33.97
6
For mixtures,
Ionization e.g.
Ar
CO
2
80 : 20
n t n p
0 .
8
0 .
8
2440
26
29 .
4
3010
0 .
2
0 .
2
33
34
30
93 /
/ cm cm
7
Charge Transfer and Recombination
Once ions and electrons are produced they undergo collisions as they diffuse/drift
These collisions can lead to recombination thus lessening the signal
8
Diffusion
Random thermal motion causes the electrons and ions to move away from their point of creation (diffusion)
From kinetic theory
3
2 kT ~ 0 .
04 eV at room temperatu re
Maxwell distributi on gives v
8 kT
m v ( electrons ) v
ions
~ 10
4
~ cm
10
6
/ s cm / s
9
Diffusion
Multiple collisions with gas atoms causes diffusion
The linear distribution of charges is Gaussian
10
Drift
In the presence of an electric field E the electrons/ions are accelerated along the field lines towards the anode/cathode
Collisions with other gas atoms limits the maximum average (drift) velocity w
11
Drift
A useful concept is mobility m
Drift velocity w = m E
For ions, w + is linearly proportional to E/P
(reduced E field) up to very high fields
That’s because the average energy of the ions doesn’t change very much between collisions
The ion mobilities are ~ constant at 1-1.5 cm 2 /Vs
The drift velocity of ions is small compared to the (randomly oriented) thermal velocity
12
Drift
For ions in a gas mixture, a very efficient process of charge transfer takes place where all ions are removed except those with the lower ionization potential
Usually occurs in 100-1000 collisions
13
Drift
Electrons in an electric field can substantially increase their energy between collisions with gas molecules
The drift velocity is given by the Townsend expression (F=ma) w
m
E
eE m t
t
N
1
v
Where t is the time between collisions, is the energy, N is the number of molecules/V and is the instantaneous velocity
14
Drift
15
Drift
Large range of drift velocities and diffusion constants
16
Drift
Note that at high E fields the drift velocity is no longer proportional to E
That’s where the drift velocity becomes comparable to the thermal velocity
Some gases like Ar-CH change with E)
4
(90:10) have a saturated drift velocity (i.e. doesn’t
This is good for drift chambers where the time of the electrons is measured
17
Drift
Ar-CO
2 is a common gas for proportional and drift chambers
18
Drift
Electrons can be captured by O by the walls
2 in the gas, neutralized by an ion, or absorbed
19
Proportional Counter
Consider a parallel plate ionization chamber of
1 cm thickness
V
Q
C
Q
0
A / d
~
100 e
10 pf
1 m
V
Fine for an x-ray beam of 10 6 photons this is fine
But for single particle detectors we need amplification!
20
Proportional Counter
C
ln
2
b / a
Close to the anode the E field is sufficiently high (some kV/cm) that the electrons gain sufficient energy to further ionize the gas
Number of electron-ion pairs exponentially increases
21
Proportional Counter
22
Proportional Counter
There are other ways to generate high electric fields
These are used in micropattern detectors
(MSGC, MICROMEGAS, GEM) which give improved rate capability and position resolution
23
Proportional Counter
Multiplication of ionization is described by the first Townsend coefficient a (E) dn
n a dx where a n
n
0 exp(
a x )
1
M
n n
0
exp r c a
a dr
a (E) is determined by
Excitation and ionization electron cross sections in the gas
Represents the number of ion pairs produced / path length 24
Proportional Counter
Values of first Townsend coefficient
25
Proportional Counter
Values of first Townsend coefficient
26
Proportional Counter
Electron-molecule collisions are quite complicated
27
Avalanche Formation
28
Signal Development
The time development of the signal in a proportional chamber is somewhat different than that in an ionization chamber
Multiplication usually takes place at a few wire radii from the anode (r=Na)
The motion of the electrons and ions in the applied field causes a change in the system energy and a capacitively induced signal dV
29
Signal Development
Surprisingly, in a proportional counter, the signal due to the positive ions dominates because they move all the way to the cathode dU
CVdV
qEdr
V
a
Na dV
q
CV
0 a
Na
CV
0 l
/
2
r dr
q l 2
ln a
Na
V
V
b
Na dV
V
q
CV
0 b
Na
CV
0 l
/
2
r dr
q l 2
ln b
Na
30
Signal Development
Considering only the ions
V
r r
dV dr dr
q l
2
ln
r dr dt
solving
m
E
for
r
l m
CV
0
2
and
1
r
substituti
a
ng
V
q
4
l
ln 1
m
CV
0 l
a
2 t
31
Signal Development
The signal grows quickly so it’s not necessary to collect the entire signal
~1/2 the signal is collected in ~1/1000 the time
Usually a differentiator is used
32
Signal Development
The pulse is thus cut short by the RC differentiating circuit
33
Gas
Operationally desire low working voltage and high gain
Avalanche multiplication occurs in noble gases at much lower fields than in complex molecules
Argon is plentiful and inexpensive
But the de-excitation of noble gases is via photon emission with energy greater than metal work function
11.6 eV photon from Ar versus 7.7 eV for Cu
This leads to permanent discharge from deexcitation photons or electrons emitted at cathode walls
34
Gas
Argon+X
X is a polyatomic (quencher) gas
CH
4
, CO
2
, CF
4
, isobutane, alcohols, …
Polyatomic gases have large number of non-radiating excited states that provide for the absorption of photons in a wide energy range
Even a small amount of X can completely change the operation of the chamber
Recall we stated that there exists a very efficient ion exchange mechanism that quickly removes all ions except those with the lowest ionization potential I
35
Gas
Argon+X
Neutralization of the ions at the cathode can occur by dissociation or polymerization
Must flow gas
Be aware of possible polymerization on anode or cathode
Malter effect
Insulator buildup on cathode
Positive ion buildup on insulator
Electron extraction from cathode
Permanent discharge
36
Gas
Polymerization on anodes
37
Proportional Counters
Many different types of gas detectors have evolved from the proportional counter
38
Drift
Ar-CO
2 is a common gas for proportional and drift chambers
39
Drift
40
Proportional Counter
41