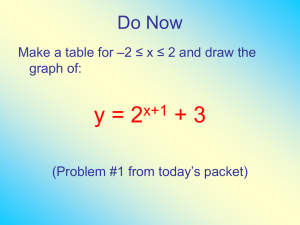
GAS TRANSFER
advertisement

GAS TRANSFER DEFINITION AND TERMS • Gas transfer a physical phenomenon, by which gas molecules are exchanged between a liquid and a gas at a gas-liquid interface (1) an increase of the concentration of the gas(es) in the liquid phase as long as this phase is not saturated with the gas under the given conditions of e.g. pressure, temperature (absorption of gas) (2) a decrease when the liquid phase is over saturated (desorption, precipitation or stripping of gas) DEFINITION AND TERMS • Important natural phenomena of gas transfer the reaeration of surface water: (1) the transfer of oxygen into surface water (2) release of oxygen produced by algal activities up to a concentration above the saturation concentration (3) release of taste and odor-producing substances (4) release of methane, hydrogen sulfide under anaerobic conditions of surface water or of the bottom deposits ELEMENTS OF AERATION AND GAS TRANSFER OPERATIONS • Gas transfer occurs only through the gas-liquid interface has to be carried out as to maximize the opportunity of interfacial contact between the two phases. • The engineering goal to accomplish the gas transfer with a minimum expenditure of initial and operational cost (energy). ELEMENTS OF AERATION AND GAS TRANSFER OPERATIONS • Four different types of aerators: (1) Gravity aerators (a) cascades the available difference head is subdivided into several steps (b) inclined planes eqipped with riffle plates to break up the sheet of water for surface renewal (c) vertical stacks droplets fall and updrafts of air ascend in counter current flow ELEMENTS -- CASCADES ELEMENTS – INCLINED PLANES ELEMENTS – VERTICAL STACKS ELEMENTS – VERTICAL STACKS ELEMENTS – VERTICAL STACKS ELEMENTS – AMMONIA STRIPPING ELEMENTS OF AERATION AND GAS TRANSFER OPERATIONS (2) Spray aerators the water is sprayed in the form of fine droplets into the air creating a large gas-liquid interface for gas transfer ELEMENTS – SPRAY AERATORS ELEMENTS OF AERATION AND GAS TRANSFER OPERATIONS (3) Air diffusers (bubble aeration) air is injected into water (a) through orifices or nozzles in the air piping system (b) through spargers (c) through porous tubes, plates, boxes or domes to produce bubbles of various size with different interfacial areas per m3 of air. ELEMENTS – AIR DIFFUSERS ELEMENTS – AIR DIFFUSERS (POROUS TUBES) ELEMENTS – AIR DIFFUSERS ELEMENTS – AIR DIFFUSERS ELEMENTS – AIR DIFFUSERS ELEMENTS OF AERATION AND GAS TRANSFER OPERATIONS (4) Mechanical aerators create new gas-liquid interfaces by different means and constructions two types of construction: (a) various construction of brushes a horizontal revolving shaft with combs, blades or angles (b) turbine or cone aerators with vertical shaft Boyle’s Law Charles’ Law V constant T (constant pressure) Gay-Lussac’s Law p constant T (constant volume) Ideal Gas Law The ideal gas law is a special form of an equation of state, i.e., an equation relating the variables that characterize a gas (pressure, volume, temperature, density, ….). The ideal gas law is applicable to low-density gases. pV T pV pV p constant (fixed mass of gas) nRT Nk BT RT Absolute Zero and the Kelvin Scale The pressure-temperature relation leads to the design of a constant-volume gas thermometer. Extrapolation of measurements made using different gases leads to the concept of absolute zero, when the pressure (or volume) is zero. Kinetic Theory: Applications Kinetic theory investigates (on a molecular scale) topics such as: •Change of phase (evaporation; vapour pressure; latent heat) •Pressure •Change of shape and volume (elasticity; Hooke's law) •Transport phenomena (diffusion - transport of mass; viscosity transport of momentum; electrical conduction - transport of electric charge; thermal conduction - transport of heat) •Thermal expansion •Surface energy and surface tension Kinetic Theory of Gases: Basic Assumptions •The number of molecules is large, and the average separation between them is large compared with their dimensions. This means that the molecules occupy a negligible volume in the container. •The molecules obey Newton's laws of motion, but as a whole they move randomly. 'Randomly' means that any molecule can move equally in any direction. •The molecules undergo elastic collisions with each other and with the walls of the container. Thus, in the collisions both kinetic energy and momentum are constant. •The forces between molecules are negligible except during a collision. The forces between a molecule are short-range, so the molecules interact with each other only during a collision. •The gas is a pure substance. All molecules are identical. SOLUBILITY OF GASES • The solubility of gases in water (and also in other liquids) depends upon: (1) the nature of the gas generally expressed by a gas specific coefficient the distribution coefficient, kD (2) the concentration of the respective gas in the gaseous phase related to the partial pressure of the respective gas in the gas phase (3) the temperature of the water (4) impurities contained in the water INFLUENCE OF THE GAS CONCENTRATION ON SOLUBILITY • The higher the gas concentration in the gaseous phase the greater will be the saturation concentration in the liquid phase • The relation between the saturation concentration cs (g/m3) and the gas concentration in the gas phase cg (g/m3): cs = kD . cg INFLUENCE OF THE GAS CONCENTRATION ON SOLUBILITY • The molar gas concentration in the gas phase (according to the universal gas law): (n/V) = p / (RT) (moles/m3) • Hence the corresponding mass concentration cg is obtained by multiplication with the molecular weight (MW) of the gas: cg = (p . MW)/ (RT) (g/m3) INFLUENCE OF THE GAS CONCENTRATION ON SOLUBILITY • The combination yields: cs = (kD . MW . p)/ (RT) • Henry’s law is generally written as: cs = kH . p • The relation between distribution coefficient kD and Henry’s constant: kH = (kD . MW)/ (RT) INFLUENCE OF THE GAS CONCENTRATION ON SOLUBILITY • Bunsen absorption coefficient, kb how much gas volume (m3), reduced to standard temperature (0oC) and pressure (101,3 kPa), can be absorbed per unit volume (m3) of water at a partial pressure of pO = 101,3 kPa of the gas in the gas phase : cs (m3 STP gas/m3 water) = kb INFLUENCE OF THE GAS CONCENTRATION ON SOLUBILITY • And any other partial pressure p: cs = kb . (p/p0) (m3STP/m3) • Since 1 m3STP contains p0/R.T0 moles of gas and a mass of gas equal to MW. p0/R.T0 : cs = (kb . MW)/(R.T0 ) p (g/m3) INFLUENCE OF THE GAS CONCENTRATION ON SOLUBILITY • The relation between kD and kb: kb = kD T0/T • The interrelationship between the three coefficients: kD = kH .R.T/MW = kb .T/T0 INFLUENCE OF THE GAS CONCENTRATION ON SOLUBILITY • In the practice of aeration the gas phase will always be saturated with water vapor exerting a certain partial pressure pw the partial pressure p of the other gases are reduced p’ = p . (P – pw)/P INFLUENCE OF TEMPERATURE ON SOLUBILITY • Gases dissolved in water accompanied by liberation of heat H • Le Chatelier principle increase of temperature results in a decrease of solubility van’t Hoff’s equation: [d(ln kD)/dT] = H/(RT2) where R = universal gas constant T = absolute temperature K H = change of heat content accompanying by the absorption of 1 mole of gas (J/mole) INFLUENCE OF TEMPERATURE ON SOLUBILITY • By integrating between the limits T1 and T2: ln[(kD)2/(kD)1]= (H/R)(T2-T1)/(T1.T2) • The product T1 .T2 does not change significantly within the temperature range encountered in gas transfer operations: (kD)2= (kD)1. econst (T2 – T1) INFLUENCE OF IMPURITIES ON SOLUBILITY • Other constituent that may be contained in water influence the solubility of gases expressed by an activity coefficient : cs = (kD/).cg • For pure water = 1 generally increases as the concentration of substances dissolved in water rises lowering the solubility INFLUENCE OF IMPURITIES ON SOLUBILITY • The influence of concentration of impurities cimp on the activity coefficient: for non-electrolytes log = f . Cimp for electrolytes log = f . I where f = a constant depending on the matter dissolved in water I = ionic strength of electrolyte DIFFUSION • The phenomenon of diffusion the tendency any substance the spread uniformly throughout the space available to it in environmental engineering diffusion phenomena the liquid phase in gas transfer operations DIFFUSION • For a quiescent body of water of unlimited depth contacting the gas by an area of A the rate of mass transfer dM/dt as a consequence of diffusion of the gas molecules in the liquid phase Fick’s Law (dM/dt) = -D.A (dc/dx) (g/s) where D = coefficient of molecular diffusion (m2/s) x = the distance from the interfacial area A dx/dt = concentration gradient DIFFUSION DIFFUSION DIFFUSION DIFFUSION • The total amount of gas M (g) that has been absorbed through the surface area A during the time t independent of x M 2 A(cs c0 ) Dt under conditions of unlimited depth of water body DIFFUSION • If the depth is not too small the time of diffusion is not too long diffusion is very slow process and only very little gas is brought into deeper layers of the water body: dM A(cs c0 ) dt D t THE CONCEPT OF GAS TRANSFER COEFFICIENTS THE CONCEPT OF GAS TRANSFER COEFFICIENTS • In accordance with Fick’s Law the mass transport per unit time (g/s) is proportional to the concentration difference : for the gas phase m k g A(cg cgi ) for the liquid phase m k L A(cLi cL ) THE CONCEPT OF GAS TRANSFER COEFFICIENTS where kg = partial gas transfer coefficient for the gas phase kL = partial gas transfer coefficient for the liquid phase cgi and cLi generally not known cLi = kD . cgi 1 k D 1 m( ) A(k D cg cL ) kL kg THE CONCEPT OF GAS TRANSFER COEFFICIENTS • The total gas transfer coefficient KL is composed of both the partial coefficients and the distribution coefficient: 1 1 kD KL kL kg then m = A KL (kDcg – cL) THE CONCEPT OF GAS TRANSFER COEFFICIENTS • The value of kD/kg will be very small with respect to 1/kL the influence of the gas transfer coefficient of the gas phase may be neglected KL = kL and consequently m = A kL (kDcg – cL) FILM THEORY FILM THEORY FILM THEORY FILM THEORY FILM THEORY PENETRATION THEORY • During the time of exposure the gas diffuses into the fluid element penetrates into liquid. • In contrast to the film theory, the penetration process is described by unsteady diffusion PENETRATION THEORY PENETRATION THEORY PENETRATION THEORY • During the time of the liquid the interface to the gas, the gases penetrate into the liquid at a diminishing rate. The total mass of gas absorbed during this time: M 2 A(k D cg cL ) Dt PENETRATION THEORY • Hence the average absorption rate m (g/s) during the time t is defined by M D m 2 A(k D cg cL ) t t The penetration assumes t =tc for a gas transfer process operated under steady state condition PENETRATION THEORY • The final form of the rate expression for gas absorption as proposed by the penetration theory: D m2 A(k D cg cL ) tc PENETRATION THEORY • According to the penetration theory: kL 2 D tc stating that the coefficient of gas transfer is proportional to the root of the coefficient diffusion. PENETRATION THEORY • Assumption of a constant time of exposure of fluid elements to the gas phase a constant rate rc (s-1) rc 1 tc • Taking rc instead of tc kL 2 Drc SURFACE RENEWAL THEORY • The model underlying the surface renewal theory is equal to that of the penetration theory unsteady diffusion of the gas into liquid elements exposed to the gas phase. • However, this theory does not assume that the time to be constant follow a frequency distribution f(t) with ages of the fluid elements (= time of exposure) ranging from zero to infinity. SURFACE RENEWAL THEORY • The theory is based on the assumption the fraction of the surface having ages between t and t+dt is given by: f (t )dt se st dt if the surface element of any age always has chance of s.dt of being replaced if each surface element is being renewed with a frequency s, independent of its age SURFACE RENEWAL THEORY • The average rate of gas transfer is • The surface renewal theory forecasts kL Ds FILM-SURFACE-RENEWAL THEORY • This theory attempts a combination of the film theory and the surface renewal theory in principle a combination of steady and unsteady diffusion. • The gas transfer coefficient as a function of the rate of surface renewal s and max x = dL kL s.d L Ds cot h D 2 COMPARISON OF THE THEORIES FACTORS AFFECTING THE GAS TRANSFER COEFFICIENTS • The effects of temperature on the rate gas transfer (effects on kL and A) A A (k L )T2 (k L )T1 . T2 T1 V V • The temperature coefficient for oxygenation of sewage in the range of 1,016 to 1,047. FACTORS AFFECTING THE GAS TRANSFER COEFFICIENTS • The influence of hydrophobic constituents and surface active agents on the rate of gas transfer Gibbs adsorption equation S c d RT dc c = concentration of hydrophobic substance in the bulk of the solution (g/m3) S = excess concentration of hydrophobic substance at the surface (g/m3) as compared with that of the bulk solution R = universal gas constant d/dc = rate of increase of surface tension with increasing the concentration of the hydrophobic substance FACTORS AFFECTING THE GAS TRANSFER COEFFICIENTS FACTORS AFFECTING THE GAS TRANSFER COEFFICIENTS THE OVERALL GAS TRANSFER COEFFICIENT OR AERATION COEFFICIENT • Under steady state conditions of gas transfer operation the coefficient diffusion and the time of exposure may be assumed constant : A D A k2 2 k L a.k L V tc V where k2 or kL.a is the overall gas transfer coefficient. THE OVERALL GAS TRANSFER COEFFICIENT OR AERATION COEFFICIENT • The rate of gas transfer can be expressed as the rate of concentration change m dc k 2 cs c V dt which integrates with c0 at t=0 to c cs cs c0 e or cs c e k 2t cs c0 k2t THE OVERALL GAS TRANSFER COEFFICIENT OR AERATION COEFFICIENT • The overall gas transfer coefficient k2 can easily determined experimentally by measuring the change of concentration as a function of time and by plotting log (cs-c)/(cs-c0) versus time : cs c log log e k2t k2t. log e cs c0 0,4343.k2t THE EFFICIENCY COEFFICIENT • With some transfer operations, e.g. cascades, weir aeration difficult or impossible to determine the parameter time t. • If now a constant time tk is assumed for the aeration step under steady state conditions: cs ce k 2t k e 1 K cs c0 cs ce c0 ce 1 1 K cs c0 cs c0 THE EFFICIENCY COEFFICIENT THE EFFICIENCY COEFFICIENT THE OXYGENATION CAPACITY THE OXYGENATION CAPACITY THE OXYGENATION CAPACITY AIR STRIPPING AIR STRIPPING AIR STRIPPING AIR STRIPPING AIR STRIPPING AIR STRIPPING AIR STRIPPING AIR STRIPPING AIR STRIPPING AIR STRIPPING AIR STRIPPING AIR STRIPPING AIR STRIPPING AIR STRIPPING