Metric conversions
advertisement

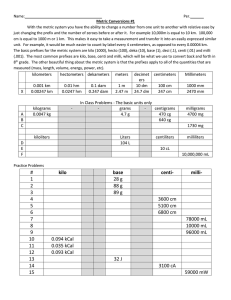
UNIT 2.2 SI UNITS/METRIC CONVERSIONS SI UNITS SI = International system of metric units. Base unit = all other units are multiples of base units or combinations) Quantity length mass time amount of substance Written unit Symbol SI UNITS 2. MULTIPLES OF BASE UNITS (p.17) Prefix Symbol Exponential mega kilo deci centi milli micro M k d c m μ 106 103 10-1 10-2 10-3 10-6 106 103 10-1 10-2 10-3 10-6 2.2 SI UNITS 3. IMPORTANT EQUIVALENCES 1 mL = 1 cm3 1 m3 = 103 L 1 t = 103 kg 2.2 SI UNITS Example: Re-write the expression “6 kilograms” The prefix symbol for kilo is ___ The unit symbol for grams is ___ 6 kilograms = The exponential equivalent to kilo is ____ 6 kilograms = METRIC CONVERSIONS Metric conversions involve using unit conversions between prefix symbols and exponential equivalents. Example: Write a conversion statement between cm and m. METRIC CONVERSIONS Write conversion statements between each of the following dm and m cL and L dmol and mol METRIC CONVERSIONS How many milligrams are in 5 kg? 5 kg = ? mg No direct link between kilo and milli What to do? Convert to a base unit first ! METRIC CONVERSIONS Express 7µm in Megametres 7µm =? Mm METRIC CONVERSIONS How many decilitres in 0.5kL? TRY 0.052 GHz = ? kHz Hz is Hertz( a frequency unit) MULTI CONVERSION Convert 1cg/ mL into dg/L MULTI CONVERSION T TRY: Convert 3 mg/L into µg/mL METRIC CONVERSIONS DERIVED QUANTITIES Derived quantity = Derived unit = DENSITY Density is an example of a derived unit What is density? Density = DERIVED UNITS: DENSITY Given that a 2.44 cm3 sample of an element has a mass of 47.14 g. Determine the density of the element. DERIVED UNITS Find the volume of a block of wood that has a mass of 34.5 grams. The density of this wood is 12g/cm3 DENSITY The density of carbon dioxide at standard temperature and pressure is 1.96 g/L. Calculate the mass of a 600 mL sample of carbon dioxide. The density of air is about 1.29 g/L at standard temperature and pressure. Would carbon dioxide tend to rise up or sink down in the atmosphere? _______________ Homework Hebden Page19 #13, 14, 15(a,c,d,f,g,i), Page 21 #16 (a,c,e) #17 (b,d,f,h,j,L,n) #18(a, b, c) Page 26 # 31,33, 37