CP504Lecture_08_OK
advertisement

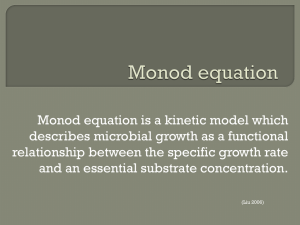
CP504 – Lecture 8 Cellular kinetics and associated reactor design: Modelling Cell Growth Approaches to modelling cell growth Unstructured segregated models Substrate inhibited models Product inhibited models 07 Oct 2011 Prof. R. Shanthini 1 Cell Growth Kinetics The most commonly used model for μ is given by the Monod model: μ= μm CS (45) KS + CS where μmax and KS are known as the Monod kinetic parameters. Monod Model is an over simplification of the complicated mechanism of cell growth. However, it adequately describes the kinetics when the concentrations of inhibitors to cell growth are low. 07 Oct 2011 Prof. R. Shanthini 2 Cell Growth Kinetics Let’s now take a look at the cell growth kinetics, limitations of Monod model, and alternative models. 07 Oct 2011 Prof. R. Shanthini 3 Approaches to modelling cell growth: Unstructured Models Structured Models (cell population is treated as single component) (cell population is treated as a multi-component system) Nonsegregated Models Segregated Models (cells are treated heterogeneous) (cells are treated as homogeneous) 07 Oct 2011 Prof. R. Shanthini 4 Approaches to modelling cell growth: Structured Segregated Models Unstructured Nonsegregated Models (cell population is treated as single component, and cells are treated as homogeneous) Simple and applicable to many situations. 07 Oct 2011 (cell population is treated as a multi-component system, and cells are treated heterogeneous) Most realistic, but are computationally complex. Prof. R. Shanthini 5 Unstructured, nonsegregated models: Monod model: μ= μm CS KS + CS Most commonly used model for cell growth μ : specific (cell) growth rate μm : maximum specific growth rate at saturating substrate concentrations CS : substrate concentration KS : saturation constant (CS = KS when μ = μm / 2) 07 Oct 2011 Prof. R. Shanthini 6 Unstructured, nonsegregated models: Monod model: μ= μm CS KS + CS Most commonly used model for cell growth 1 μ (per h) 0.8 0.6 0.4 μm = 0.9 per h Ks = 0.7 g/L 0.2 0 07 Oct 2011 0 5 Prof. R. Shanthini 10 Cs (g/L) 15 7 Assumptions behind Monod model: - One limiting substrate - Semi-empirical relationship - Single enzyme system with M-M kinetics being responsible for the uptake of substrate - Amount of enzyme is sufficiently low to be growth limiting - Cell growth is slow - Cell population density is low 07 Oct 2011 Prof. R. Shanthini 8 Other unstructured, nonsegregated models (assuming one limiting substrate): Blackman equation: μ = μm if CS ≥ 2KS μ = Tessier equation: Moser equation: Contois equation: 07 Oct 2011 μm CS 2 KS if CS < 2KS μ = μm [1 - exp(-KCS)] μ = μ = μm CSn KS + CSn μm CS KSX CX + CS Prof. R. Shanthini 9 Blackman equation: μ = μm μ= μm CS 2 KS if CS ≥ 2 KS if CS < 2 KS This often fits the data better than the Monod model, but the discontinuity can be a problem. 1 μ (per h) 0.8 0.6 μm = 0.9 per h Ks = 0.7 g/L 0.4 0.2 0 07 Oct 2011 0 Prof. R. Shanthini 5 Cs (g/L) 10 10 Tessier equation: μ = μm [1 - exp(-KCS)] 1 μ (per h) 0.8 0.6 μm = 0.9 per h K = 0.7 g/L 0.4 0.2 0 07 Oct 2011 0 2 Prof. R. Shanthini 4 6 Cs (g/L) 8 11 10 Moser equation: μ = μm CSn When n = 1, Moser equation describes Monod model. KS + CSn 1 μ (per h) 0.8 0.6 0.4 μm = 0.9 per h Ks = 0.7 g/L 0.2 Monod n = 0.25 n = 0.5 n = 0.75 0 07 Oct 2011 0 2 Prof. R. Shanthini 4 6 Cs (g/L) 8 12 10 Contois equation: μ = μm CS Saturation constant (KSX CX ) is proportional to cell concentration KSX CX + CS 07 Oct 2011 Prof. R. Shanthini 13 Extended Monod model: μ= Extended Monod model includes a CS,min term, which denotes the minimal substrate concentration needed for cell growth. μm (CS – CS,min) KS + CS – CS,min 1 μ (per h) 0.8 0.6 μm = 0.9 per h Ks = 0.7 g/L CS,min = 0.5 g/L 0.4 0.2 0 07 Oct 2011 0 Prof. R. Shanthini 5 Cs (g/L) 10 14 Monod model for two limiting substrates: μ = μm 07 Oct 2011 CS1 CS2 KS1 + CS1 KS2 + CS2 Prof. R. Shanthini 15 Monod model modified for rapidly-growing, dense cultures: Monod model is not suitable for rapidly-growing, dense cultures. The following models are best suited for such situations: μ= μ= μm CS KS0 CS0 + CS μm CS KS1 + KS0 CS0 + CS where CS0 is the initial substrate concentration and KS0 is dimensionless. 07 Oct 2011 Prof. R. Shanthini 16 Monod model modified for substrate inhibition: Monod model does not model substrate inhibition. Substrate inhibition means increasing substrate concentration beyond certain value reduces the cell growth rate. 1 μ (per h) 0.8 0.6 0.4 0.2 0 07 Oct 2011 0 Prof. R. Shanthini 5 Cs (g/L) 10 17 Monod model modified for cell growth with noncompetitive substrate inhibition: μ= = μm (1 + KS/CS)(1 + CS/KI ) μm CS KS + CS + CS2/KI + KS CS/KI If KI >> KS then μ= μm CS KS + CS + CS2/KI where KI is the substrate inhibition constant. 07 Oct 2011 Prof. R. Shanthini 18 Monod model modified for cell growth with competitive substrate inhibition: μ= μm CS KS(1 + CS/KI) + CS where KI is the substrate inhibition constant. 07 Oct 2011 Prof. R. Shanthini 19 Monod model modified for cell growth with product inhibition: Monod model does not model product inhibition (where increasing product concentration beyond certain value reduces the cell growth rate) For competitive product inhibition: μm CS μ= KS(1 + Cp/Kp) + CS For non-competitive product inhibition: μm μ= (1 + KS/CS)(1 + Cp/Kp ) where Cp is the product concentration and Kp is a product inhibition constant. 07 Oct 2011 Prof. R. Shanthini 20 Monod model modified for cell growth with product inhibition: Ethanol fermentation from glucose by yeasts is an example of non-competitive product inhibition. Ethanol is an inhibitor at concentrations above nearly 5% (v/v). Rate expressions specifically for ethanol inhibition are the following: μ= μ= μm CS (KS + CS) μm CS (KS + CS) (1 + Cp/Cpm) exp(-Cp/Kp) where Cpm is the product concentration at which growth stops. 07 Oct 2011 Prof. R. Shanthini 21 Monod model modified for cell growth with toxic compound inhibition: For competitive toxic compound inhibition: μ= μm CS KS(1 + CI/KI) + CS For non-competitive toxic compound inhibition: μ= μm (1 + KS/CS)(1 + CI/KI ) where CI is the product concentration and KI is a constant to be determined. 07 Oct 2011 Prof. R. Shanthini 22 Monod model extended to include cell death kinetics: μ= μm CS KS + CS - kd where kd is the specific death rate (per time). 07 Oct 2011 Prof. R. Shanthini 23 Beyond this slide, modifications will be made. 07 Oct 2011 Prof. R. Shanthini 24 Other unstructured, nonsegregated models (assuming one limiting substrate): Luedeking-Piret model: rP = rX + β CX Used for lactic acid formation by Lactobacillus debruickii where production of lactic acid was found to occur semiindependently of cell growth. 07 Oct 2011 Prof. R. Shanthini 25 Modelling μ under specific conditions: There are models used under specific conditions. We will learn them as the situation arises. 07 Oct 2011 Prof. R. Shanthini 26 Limitations of unstructured non-segregated models: • No attempt to utilize or recognize knowledge about cellular metabolism and regulation • Show no lag phase • Give no insight to the variables that influence growth • Assume a black box • Assume dynamic response of a cell is dominated by an internal process with a time delay on the order of the response time • Most processes are assumed to be too fast or too slow to influence the observed response. 07 Oct 2011 Prof. R. Shanthini 27 Filamentous Organisms: • Types of Organisms – mold – bacteria or yeast entrapped in a spherical gel particle – formation of microbial pettlets in suspension • Model - no mass transfer limitations dR k const dt where R is the radius of the cell floc or pellet or mold colony 07 Oct 2011 Prof. R. Shanthini 28 Filamentous Organisms: Then the growth of the biomass (M) can be written as dM 2 dR 4R k p 4R 2 dt dt or dM M 2 / 3 dt where 07 Oct 2011 k p (36) 1/ 3 Prof. R. Shanthini 29 Filamentous Organisms: • Integrating the equation: 1/ 3 t t M M0 3 3 3 3 • M0 is usually very small then M t • Model is supported by experimental data. 3 07 Oct 2011 Prof. R. Shanthini 30 Chemically Structured Models : • Improvement over nonstructured, nonsegregated models • Need less fudge factors, inhibitors, substrate inhibition, high concentration different rates etc. • Model the kinetic interactions amoung cellular subcomponents • Try to use Intrinsic variables - concentration per unit cell mass- Not extrinsic variables - concentration per reactor volume • More predictive • Incorporate our knowledge of cell biology 07 Oct 2011 Prof. R. Shanthini 31