UniMasr.com_0773e513110a63ba6d0a7422f57e8af6_1
advertisement

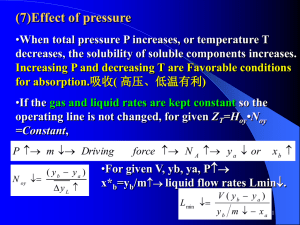
Absorption Prepared by Dr.Nagwa El-Mansy Cairo University Faculty of Engineering Chemical Engineering Department Forth year References:1-Coluson and Richerdson, Chemical Engineering vol , vol II , vol III. 2- Geancoplis, Principles of Unit Operation. 3- Mc-Cabe and Smith, Unit operations for Chemical Engineering. 4- Traybal, Mass Transfer Operations. 5- Sherwood, Mass Transfer. 6-Perry’s , Chemical Engineering. 7- “Separation Process Principles”, 2nd ed, Seader et’al . 8- Site on Google search, Separation Processes. Absorption Absorption:It is a gas-liquid mass transfer operation in which liquid solvent is contacted with gas mixture for differential dissolution of one or more components of gas and provide a solution of them in liquid. Uses of absorption:1- Purification of gases (H₂S from HC’s). 2-Separation of gases (separation of dry gas [C₁,C₂] from LNG [C₃,C₄]. 3- Production of useful liquid product:HCL (g) + H₂O (liq) → HCL (liq) 2NO₂(g) + H₂O (liq) → HNO₃ + HNO2 SO₃ (g) + H₂O (liq) → H₂SO₄ Applications of absorption: 1- Hydrogen sulfide(H2S) is removed from hydrocarbon gases by washing with alkaline solution (Amines). 2- Washing ethanol vapors from carbon dioxide from molasses fermentor tanks with water to remove ethanol. 3- Acetone can be recovered from acetone-air mixture by passing the gas stream into water in which acetone is dissolved while air is passed out. 4-Carbon dioxide present in air is absorbed by sodium hydroxide (NaOH solution) in which chemical absorption takes place. 5- Nitrogen oxides are absorbed in water to give nitric acid. 6- Removal of ammonia coming from coke ovens by water Choice Of Solvent For Gas Absorption The factors to be considered are High absorption power Which means that gas solubility should be high in the solvent, which results in increasing the rate of absorption and decreasing the quantity of solvent required. Highly Selective The selectivity of solvent must be high in which solvent dissolve one and leave the others. Easy to recover Which means easily to be regenerated. Low volatility The solvent should have a low vapor pressure to reduce loss of solvent in the gas leaving the absorption column. Small viscosity Low viscosity is preferred for reasons of rapid absorption rates, improving flooding characteristics in packed column, low pressure drops on pumping and good heat transfer characteristics. Cost The solvent should be inexpensive, so that losses are not costly, and should be readily available. Other properties Non-toxic, Non-flammable, Non-corrosive, Chemically stable, low freezing point Absorption Equipments (A) Plate Towers:1-Multistage contact. 2-High separation , high capacity. 3-Relatively large diameter. 4-Cooling is done by providing the plate with cooling coils. 5- High pressure drop. 6- Easy to be clean. (B) Packed Columns 1-Differential contact. 2-Used for highly corrosive materials. 3- Small diameters <70-80 cm 4-Not easy to clean. 5-Packing materials are made from(ceramics , bricks, wood, gravels, stones , steel ,……) 6-To increase surface area of contact between the two phases in packed columns, make more than one section which increase the performance of the tower. 7- cooling is done by dividing the column To many sections out side the column (as seen in the opposite Figure). (C) Spray Column:1- Continuous contact. 2- Low pressure drop. 3- Low efficiency. 4- Low cost(empty). 5- Gas phase controlling. 6- Considered as one stage. (D) Wetted wall Column:Single tube wetted wall column used in labs for measuring mass transfer coefficient. (E) Tubular Reactor:1- used for highly exothermic reactions. 2-for highly heats of reactions. 3- proper for heat transfer control. 4- low mass transfer due to small surface area of contact. (F) U-Tube Absorber:1- Specially for highly corrosive materials(HCL) 2- Small surface area of contact between two phases. 4-Simple in construction. 5-Use any material of construction( ceramic, cast iron, silicon,……)to over come corrosion problems. 6-Very difficult in casting and welding. (G)Centrifugal type of absorption:1- Single stage absorber. 2-Co-current contact 3-Used for highly viscous liquids. 4-Used for foamy liquids. 5-Liquids are sprayed by centrifugal force. 6-Provied good contact between two phases. 7-Operating and initial cost are very high. Equilibrium Relations:Mass transfer between G/L depends highly on the equilibrium between G/L. Different gases and liquids yield separate solubility curves , which must be determined experimentally for each system. If the equilibrium pressure of a gas at a given liquid concentration is high, as case (A) in the opposite figure, the gas is said to be relatively insoluble in liquid , while if its low, as for curve (B) , the solubility is said to be high. Effect of temperature on the equilibrium curve:The solubility of any gas is influenced by the temperature. If the temperature of the system at equilibrium is raised , the solubility of a gas decreases . As shown in the opposite figure as temperature increases for the same solute (gas) the solubility decreases from (1060)oC and the absorption power decreases . Absorption process is usually accompanied by evolution of heat. So It is necessary to fit coolers to the absorber to keep its temperature sufficiently low. Effect of temperature on the equilibrium curve Types of Equilibrium Relations :For dilute concentrations of many gases the equilibrium relationship is given by Henry’s law which relates the partial pressure developed by a dissolved solute(A) in a liquid solvent (S) by the following equation:PA = H xA Where:H is Henry’s constant expressed as kPa / mole fraction solute in liquid, PA is the partial pressure of solute in kPa, xA is the mole fraction of the gas in liquid phase Henry’s law holds very well when the partial pressure of the solute is less than atmospheric. Above atmospheric pressure , H may be independent of the partial pressure. The variation of H with temperature is strongly nonlinear function. For ideal systems Raoult’s law is valid:PA = PoA xA Where PA , is the partial pressure of solute . PoA , is the vapor pressure of solute. xA , is the mole fraction of the solute in the liquid phase. PA = H A x A PA HA x A = PT PT (by dividing each tearm by PT ) y A = m* x A (where x A andy A are mole fractions) Conversion from mole fraction to mole ratio:mole fraction of A in gas phase y A = nA nA + nB mole fraction of A in liquid phase x A = nA n A + nS nA mole ratio of A in gas phase y A = nB , mole ratio of A in liquid phase x A = nA nS , PA = H A x A nA m* n A = nA + nB n A + nS m*n A /n S n A /n B = n A /n B +n B /n B n A /n S +n S /n S YA m* X A = YA +1 X A +1 YA m*X A = YA +1-YA X A +1- m*X A m*X A YA X A +1- m*X A or YA m*X A (an equilibrium relation at certain temperature and pressure) X A (1- m*)+1 Factors affecting absorption process:A- Choice of solvent flow rate :Usually given:1- gas flow rate(Gin). 2- feed composition(yin). 3-solvent composition( xin ). 4-degree of separation= sharpness of separation. recovery= (Yin – Yout )/ Yin in which we can calculate the outlet gas composition. Yout = Yin (1 – recovery) Here we want to calculate proper solvent rate(Lin) By using mole or mass ratios we must remove the amounts of solute from gas and liquid flow rates as flows:Ginert = G’ = G in ( 1 – yin) . Where yin is feed mole fraction L inert = L’ = L in ( 1 – x in). Where xin is solvent mole fraction By making material balance on the absorber: G’ Yin + L’ Xin = G’ Yout + L’ Xout G’ ( Yin – Yout ) = L’ ( Xout – Xin) L’ / G’ = (Yin – Yout) / (Xout – Xin) [ Operating line equation] Operating line is a line between two points (Xin,Yout) and (Xout, Yin) and has a slope - L’/G’ As the amount of solvent decreases (L’) the slope of the operating line decreases and goes down and the number of stages increases. The effect liquid amount on the number of stages:As the amount of liquid solvent decreases the driving force decreases and the number of stages increases thus the tower cost increases till operating line cuts or touch the equilibrium curve at this point we reach pinch point which means no separation. But if we increase the amount of liquid solvent ,the slope of the operating line goes up and the driving force increases which means small number of stages is required( also small number of transfer units). This means that we must make optimization for liquid amount as shown in the opposite figure. We have to use L/G > ( L/G)Min (L/G) Opt =[ 1.2 to 2.5 ] (L/G) Min (B)Temperature:In general absorption process is an exothermic process , it improves by lowering temperature. Thus we make good cooling for liquid solvent before entering the column. Increasing temperature results in:1- equilibrium curve goes up and absorption power decreases. Notice that for the same Y the separation increases with decreasing temperature as shown in the opposite Figure. X3 < X2 < X1 2-For same (L/G), Number of stages (or NTU) increases , means tall column and high cost ,which is bad conditions. 3-for same(L/G) , driving force decreases and separation becomes difficult ,which is a bad conditions In some cases even refrigeration is economic, this happen when losses in solvent is high ( to minimize losses = economic). Some times average slight increase in temperature is permissible and have +ve effect when:1- Solvent has high viscosity. 2- Case of chemical reaction, in which rate of absorption is affected positively by increasing temperature. The highest temperature (T Max) in the absorber can be found at the bottom of the column. (C)Pressure:As pressure increases absorption power increases PA = HA xA( Henry’s law) PA / PT = (HA / PT) xA y A = m* xA Increasing pressure results in:1- the equilibrium curve goes down which improves the absorption process. Notice that for the same (Y) the separation increases with increasing pressure as shown in the opposite figure X3 < X2 < X1 2-For same (L/G), Number of stages (or NTU) decreases ,means short column and low cost which is good conditions 3-Driving force increases and separation becomes more easier which means good separation. Physical vs chemical absorption:There are 2 types of absorption processes: physical absorption and chemical absorption, depending on whether there is any chemical reaction between the solute and the solvent (absorbent). When water and hydrocarbon oils are used as absorbents, no significant chemical reactions occur between the absorbent and the solute, and the process is commonly referred to as physical absorption. When aqueous sodium hydroxide (a strong base) is used as the absorbent to dissolve an acid gas, absorption is accompanied by a rapid and irreversible neutralization reaction in the liquid phase and the process is referred to as chemical absorption or reactive absorption. More complex examples of chemical absorption are processes for absorbing CO2 and H2S with aqueous solution of mono ethanolamine (MEA), di -ethanolamine (DEA), diethyleneglycol (DEG) or tri-ethyleneglycol (TEG), where a reversible chemical reaction takes place in the liquid phase. Chemical reactions can increase:1- the rate of absorption. 2- increase the absorption capacity of the solvent. 3-increase selectivity to a certain components of the gas, and convert a hazardous chemical to a safe compound. Physical absorption:- Chemical absorption:- A solute of gas (A) is absorbed from a mixture by solvent liquid(B), which combines with (A) according to the equation A + B→ AB. As the gas approaches the liquid interface, it dissolves and reacts at once with (B). The new product(AB),thus formed , diffuses towards the main body of the liquid. The concentration of (B) at the interface falls; this results in diffusion of (B) from the bulk of the liquid phase to the interface. Since the chemical reaction is rapid,(B) is removed very quickly, so that it is necessary for the gas (A) to diffuse through part of the liquid film before meeting (B). There is a zone of reaction between A and B which moves away from the gas-liquid interface. The final position of this reaction zone will be such that the rate of diffusion of (A) from the gas-liquid interface is equal to the rate of diffusion of (B) from the main body of the liquid. Eight distinct kinetic regimes are observed. For instantaneous , reaction or for rapid chemical reaction, the reaction occurs only in liquid film during the transportation of component(A). The concentration of (A) in the bulk of the liquid is zero(rate of reaction of A ((rA)=0), such as absorption of acid gas. These reactions are characterized by Hatta number (Ha) :Ha = max possible conversion in liquid film/max diffusion transport through the liquid film = (K’ CBo δ2L /DA)> 3 where:- K’ = the reaction constant. CBo = liquid concentration δ2L = liquid film thickness DA = diffusivity of solute A At the other extreme, for very slow chemical processes occurs in the liquid bulk no reaction occurs in the film and mass transfer is used to keep the bulk concentration of component (A) close to the saturation value ( CA = CA*). These reactions are characterized by Ha<< 1 such as oxidation, hydrogenation. Notice that chemical reaction affect the equilibrium curve. Effect of temperature on the absorption tower:Many absorbers and strippers deal with dilute gas mixtures and liquids, in these cases it’s assumed that the operation is isothermal. But actually absorption operations are usually exothermic, and when large quantities of solute gas are absorbed to form concentrated solutions, the temperature effects cannot be ignored. If by absorption the temperature of the liquid is raised to a considerable extent, the equilibrium solubility of the solute will be appreciably reduced. Cooling must be done to over come the increase in liquid temperature. Consider the tray tower shown in the figure. If Qc is the heat removed per unit time from the tower by any means. Enthalpy balance :For non-adiabatic operation:G ( Yin – Yout) q = L cp ( Tout - Tin ) + Qc . For adiabatic operation. G (Yin –Yout) q = L cp (Tout -Tin ) Cp = specific heat for pure liquid. q = heat of absorption (J/mole). By studying the adiabatic operation there will be some assumptions must be considered:1-No heat is removed inside the tower, Qc= zero. 2-All the amount of heat due to absorption increase the liquid temperature only. 3-No evaporation in the liquid solvent (no losses). To estimate the temperatures inside the absorber, the heat balance equation to compute the temperature of the liquid leaving each plate from the top to the bottom ,is as shown in the opposite figure:Section (1):L ( X1 - Xin) q = L Cpliq (Tout 1 -Tin ) X1 – Xin = [Cp liq /q ] ( Tout 1 - Tin ) Section (2):L ( X2 – Xin ) q = L Cp liq ( Tout 2 – T in ) X2 – X in = [Cp liq / q] ( Tout 2 – T in ) After calculating X1 , X2 , X3 ,……., we must plot new equilibrium curve differ than the case of isothermal absorption. Multi- component absorption:(A) Graphical method:The procedures for multicomponent absorption are identical for binary mixtures. Instead of having a single equilibrium curve and operating line, there are now an equilibrium curve and operating line for each absorbed component of the gas. Gas flow rate(G) and liquid flow rate(L) are approximately constant through the column. The operating line is located with the point (xin,yout) and the slope (L/G) for the key component(the component which has more data), and because the feed composition is known we can locate the terminal point of the operating line (xout,yin). Now the number of stages required for specified recovery can be determined by stepping-off stages from the other end . Exactly the same number of stages are available for the other components. Also the operating lines must have the same slope. Thus we can calculate the recovery for each entering component. Equilibrium relations may be based on the mole fraction (B) Analytical method:Kremser equation represents an analytical solution to a classical separation problem of N ideal equilibrium stages concerned with countercurrent gas and liquid flow. The equilibrium and operating relations are assumed to be linear. By using the data of the key component and by calculating the absorption factor (A=L/m*G) we can calculate number of stages from the following chart. After calculating N we can calculate the recovery for each other component. Stripping (desorption):Stripping is the opposite of absorption and involves the removal of dissolved gases in liquid by stripping agent. Purpose of stripping:1- recover the dissolved solute. 2- recover the solvent. 3-to recover both solute and solvent. Usually absorption is followed by stripping or desorption. The most commonly used stripping agent is steam. Good stripping agent must be:1- easily condensed. 2- easily separated from the material stripped. Equilibrium relations:(As absorption) The following points must be taken into consideration:1-Operating line is under the equilibrium curve. 2- slope of the operating line = L/G= (Y’in-Y’out / X’out-X’in ) 3- As (L/G) decreases, G increases , operating line goes down, driving force increases, N decreases and NTU decreases. 4-As (L/G) increases, G decreases, operating line goes up, driving force decreases, N increases and NTU increases, till we reach pinch point. Special types of absorbers:(1) Absorber with reboiler (combined absorber/stripper) When wet gas( C2H6/ C3H8/C4 H10) is contacted with oil solvent it dissolve small amount of C2H6. C2H6 can be concentrated in the gas stream leaving the column by heating the rich liquid oil stream to strip out C2H6. (2)Absorption with two solvents Recovery of highly volatile solvent e.g. recovery of C5 from C3 and C4. Solvent should have low vapor pressure to minimize losses in it’s amount. A second less volatile solvent (Kerosene)can be used to recover the evaporated portion of the first solvent (Benzene).