powder-properties-05..
advertisement

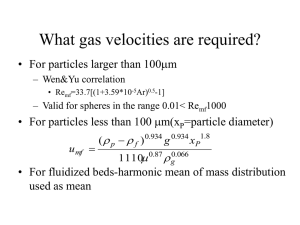
Behavior of Powders - Outline • Interparticle Forces – – – – Van der Waals Forces Adsorbed Liquid Layers & Liquid bridges Electrostatic Solid Forces • General Classifications for Fluidized Beds van der Waals • Weakest force exists between solids; is of molecular origin • For the case of a sphere near a wall R y FVW KH R 6 y2 KH: Hamaker constant (varies with material) Between two flat surfaces FVW 1 6 y y Particles & Liquids • If particles are present with a condensable vapor, the surface may have a layer of condensed vapor on it This bond may be stronger than bare surface van der Waals forces • Adsorbed liquid can smooth over defects increasing contact area • More liquid leads to liquid bridges Types of Liquid Bonding a) b) c) d) Pendular-looks like bridge, but particles not immersed in liquid Funicular-thicker bridges but not completely filled Capillary-particles at edge of cluster not completely wetted by liquid Droplet-all particles completely wet Pendular- a closer look Pc: pressure inside capillary liquid 1 1 PC r1 r2 • • • • When Pc<PA, particles will want to come together Surface tension forces always pull particles together This arrangement creates strongest interparticle bond With more liquid, particles can move more freely Electrostatic & solid Bridges • Same as for aerosols, charged powders can repel each other • Solid bridges-imagine liquid above was NaCl/water • If powder in dried crystallites of salt would remain holding particles together • Other compounds called binders (liq. or solid form) can be used by dissolving in liquid & drying • Solid binders –another type, dry powders that react with liquid to form solid bridges Interparticle Forces are functions of: • • • • • Particle size Liquid concentration Humidity Temperature Interrelationship of above variables Behavior of Particles in Fluidized Beds • Depending on particle characteristics and interparticle forces, fluidization behavior differs • Group A- can be fluidized by air at ambient conditions(least cohesiveness) over a range of fluidization velocity • Group B- powders that bubble under some conditions where Group A would not bubble (more cohesive) • Group C- fine powders that cannot be fluidized without bubbling(even more cohesive) • Group D- large powders that form spouting beds(coarse powders, may have low cohesivity) Flow in Packed Beds (not fluidized) • Darcy’s rule for laminar flow P u H for case of randomly packed bed of monosized particles More exactly u: superficial velocity through bed H: bed thickness P: pressure drop • • (diameter=x) , where =void fraction, =fluid viscosity (P) u (1 ) 180 For turbulent flow ( =fluid density) 2 3 H x 2 f (P) u (1 ) 1.75 f 3 H x Criteria & overall expression • Packed Bed Reynolds # – Laminar Re*<10 – Turbulent Re*>2000 Re * xu f (1 ) • General eq’n.=Ergun eq’n P 150u (1 ) 2 u 2 (1 ) 1.75 f 2 3 H x x 3 Pressure drop for non spherical Particles • For laminar flow (xsv=surface-volume mean diameter) (P) u (1 ) 2 180 2 3 H x sv – xsv=sphere having same surface to volume ratio as particles need mean if particles are not uniform • For entire range of Re* P 150u(1 ) 2 u 2 (1 ) 1.75 f 2 3 3 H x sv x sv Friction Factors-Packed Beds 3 P x • f*=friction factor= 2 H u ( 1 ) f * • In terms of Re laminar f*=150/Re*+1.75 • Three regimes Laminar f*=150/Re* Turbulent f*=1.75 turbulent logf* f* constant! Log Re Fluidization: backwards packed bed • When upwards drag exceeds apparent weight of particles bed becomes fluidized force balanceon particles unit area • F=gravity-upthrust pressure drop gravity P HA Upwards drag u • (1 )( p f ) A H (1 )( p forces f )g This eq’n ignores interparticle g Fluidization-Relationship between P & u Pip Fluidized bed region Minimum fluidizatio nvelocity • Pip=related to extra forces needed to overcome interparticle forces Dimensionless numbers • Ar=Archimedes # f ( p f ) gx 2 3 sv • Gravity & buoyancy vs. viscous forces • Remf=Reynolds# at incipient fluidization umf xsv f Fluidized Bed vocabulary • Mass of particles in bed=MB=(1-)PAH A:area (cross section) of bed H: bed height P:particle density :void fraction Absolute density= mass of particle volume of solidsmaterial making up the particle mass of particles in a bed volumeof particles& voids bet ween them Bed density= Bulk density= mass of particles volumeof particles& voids bet ween them