File
advertisement

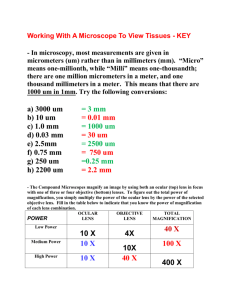
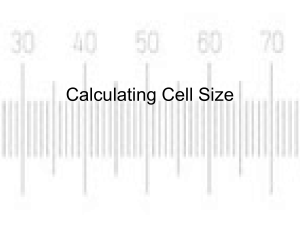
Lab 1: Using the Microscope Biology 2201, Unit 1 Safety in the Lab Read and heed the Laboratory Safety guidelines on pages x to xi in the McGraw-Hill Ryerson Biology text. Read the 1st Lab on pages 15 to 19 and page 24 in the text. Familiarize yourself with the locations of the safety equipment in the Science Lab. Magnification To calculate the total magnification of an object, multiply the power of the eyepiece by the power of the objective. For example, if the eyepiece magnification is 10x and the low-power objective is 4x, the total magnification of the low-power objective is 10 x 4 = 40x. Illumination The light source (electric bulb) directs light through the diaphragm, the specimen and the lens. Look through the eyepiece, and adjust the diaphragm until the view is as bright as possible. Field of view The area that you can see through the eyepiece is called the field of view. To determine the field of view, place a clear plastic ruler on the stage. With the low-power objective in place, use the coarse adjustment knob to focus on the ruler. Position the ruler so that one of the millimetre markings is at the left edge of the field of view. Calculate the size of your F.O.V. The diameter of the field of view under low-power, shown here, is about 1.5 mm. If you know the diameter of the field of view for the low-power lens, you can calculate the field of view for the medium-power lens as follows (example only): Med-power FOV = Low-power FOV x Magnification of low-power objective Magnification of med-power objective = 2 mm x 4X 10X = 2 mm x 0.4 = 0.8 mm or 800 µm is the Med-power FOV Formula for calculating the high-power FOV High-power FOV = Low-power FOV x Magnification of low-power objective Magnification of high-power objective Measurement Objects in the field of view of a microscope are usually measured in micrometres (µm). For example: 1 µm equals 0.001 mm 1000 µm equals 1 mm From our example, the FOV under the medium-power objective is 0.8 mm x 1000 = 800 µm. Calculating specimen size You can determine the size of a specimen, such as a microscopic amoeba, by estimating how many could fit end to end across the field of view. To do this, divide the field of view by the number of specimens. For example, if the field of view in the illustration is 1500 µm, what is the diameter of each amoeba? Answer: Specimen size = FOV divided by # of specimen = 1500 µm divided by 5 = 300 µm is the diameter of an amoeba. Focusing techniques The low-power objective should be in position. Center the specimen over the opening in the stage. Look through the eyepiece. Slowly turn the coarse adjustment knob until the object is in focus. Carefully rotate the revolving nosepiece to the medium-power objective. Use the fine adjustment knob to sharpen the focus. Watch the side of the stage as you rotate the nosepiece to make sure that the objective lens does not strike the surface of the slide. Adjust the focus using only the fine adjustment knob. To finish, carefully rotate the nosepiece until the lowpower objective is in place; remove slide; power off. Biological drawings Biological drawings are drawings of biological specimens that you observe under the microscope. A scale drawing is a drawing in which the proportions of what you observe through a microscope are kept constant. Label your drawing. Indicate the total magnification. Calculate the actual size of the specimen. Depth of field The depth of field is the vertical distance (the “thickness” of an object) that remains in focus at any one time while the specimen is being viewed. Video link Preparing a wet mount Start with a clean slide and coverslip. Hold the slide and coverslip by their edges to avoid getting your fingerprints on their surfaces. Using tweezers, position the specimen in the centre of the slide. With the medicine dropper, place one drop of water on the specimen. Hold a coverslip over the sample at a 45° angle. One edge of the coverslip should touch the surface of the slide near the specimen. Lower the opposite edge of the coverslip over the sample. Make sure that no bubbles form beneath the coverslip. Next day we meet in the Lab. Be prepared!