Why do clouds form?
advertisement

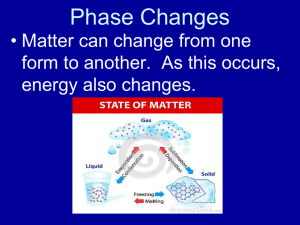
Why do clouds form? The physics behind the phenomenon Pictures and presentation by Andrew J. Orgonik Plainedge Middle School We have all looked up at clouds… …and maybe even gazed down on them from above! They add a dynamic view to the landscape… …can make an ordinary sunset amazing! …and are omens of bad weather ahead. What are the “ingredients” for cloud formation? And how do all of these components come together to create the clouds we see almost everyday?! Ingredients Required for Clouds: Water vapor (water as a gas) Conditions favoring the change of state (from gas to liquid or ice) A surface for water vapor to condense on (condensation nuclei) Why do we have clouds composed of water when it makes up less than 5% of our atmosphere, and is some areas is less than 1%? As we already know, nitrogen (78% by volume) and oxygen (21%) are the dominant gases in our troposphere. …why don’t we have nitrogen and oxygen clouds??? Water can exist at all states, and change state at temperatures experienced on the Earth’s surface and lower atmosphere Also a liquid! Water vapor: gas (invisible) ice liquid Conversely, nitrogen and oxygen are not liquid at Earth temperatures! Must be cooled to –196o C to condense! (liquefy) Must be cooled to –183o C to condense! (liquefy) As clouds are composed of tiny liquid droplets, nitrogen and oxygen clouds are impossible in the Earth’s troposphere. How does water vapor get into the air in the first place? T E E T T By evaporation and transpiration E Evaporation requires energy Some liquid water molecules gain more energy than the surrounding liquid molecules These molecules can now change into the gas phase (evaporate) because they can break free of the attractive forces from the other liquid molecules The liquid molecules left behind have less energy Therefore evaporation is a cooling process! The GREATER the temperature, the greater the evaporation rate (more molecules of liquid have the energy needed to “escape”) This basic idea is really important for understanding how clouds form! Water vapor exerts a pressure called “vapor pressure.” This is a fundamental for understanding cloud formation! Pressure gauge In a closed container of water, molecules of liquid evaporate and change into a gas. Pressure gauge In a closed container of water, molecules of liquid evaporate and change into a gas. Pressure gauge In a closed container of water, molecules of liquid evaporate and change into a gas. Pressure gauge In a closed container of water, molecules of liquid evaporate and change into a gas. A small increase in pressure will be detected due to the addition of motion of water molecules in the air above. Pressure gauge In the atmosphere, this is called vapor pressure: “The part of the total atmospheric pressure attributable to its water vapor content.” Pressure gauge At first (in a sealed container), more molecules of water will leave the water’s surface (evaporate) than will return (condensation) Pressure gauge At first (in a sealed container), more molecules of water will leave the water’s surface (evaporate) than will return (condensation) Pressure gauge At first (in a sealed container), more molecules of water will leave the water’s surface (evaporate) than will return (condensation) Pressure gauge At first (in a sealed container), more molecules of water will leave the water’s surface (evaporate) than will return (condensation) Pressure gauge At first (in a sealed container), more molecules of water will leave the water’s surface (evaporate) than will return (condensation) Pressure gauge At first (in a sealed container), more molecules of water will leave the water’s surface (evaporate) than will return (condensation) Pressure gauge However, as more and more molecules evaporate from the water’s surface, the steadily increasing vapor pressure in the air above forces more and more vapor molecules to return to the liquid. Pressure gauge However, as more and more molecules evaporate from the water’s surface, the steadily increasing vapor pressure in the air above forces more and more vapor molecules to return to the liquid. Pressure gauge However, as more and more molecules evaporate from the water’s surface, the steadily increasing vapor pressure in the air above forces more and more vapor molecules to return to the liquid. Pressure gauge However, as more and more molecules evaporate from the water’s surface, the steadily increasing vapor pressure in the air above forces more and more vapor molecules to return to the liquid. Pressure gauge However, as more and more molecules evaporate from the water’s surface, the steadily increasing vapor pressure in the air above forces more and more vapor molecules to return to the liquid. Pressure gauge Eventually, a balance is reached in which the number of vapor molecules returning to the surface balances the number of liquid molecules leaving the surface. Pressure gauge Eventually, a balance is reached in which the number of vapor molecules returning to the surface balances the number of liquid molecules leaving the surface. Pressure gauge Eventually, a balance is reached in which the number of vapor molecules returning to the surface balances the number of liquid molecules leaving the surface. Pressure gauge Eventually, a balance is reached in which the number of vapor molecules returning to the surface balances the number of liquid molecules leaving the surface. Evaporation = Condensation! Pressure gauge Eventually, a balance is reached in which the number of vapor molecules returning to the surface balances the number of liquid molecules leaving the surface. Evaporation = Condensation! Pressure gauge Eventually, a balance is reached in which the number of vapor molecules returning to the surface balances the number of liquid molecules leaving the surface. Evaporation = Condensation! Pressure gauge This condition is often referred to as “saturation vapor pressure.” However, “equilibrium vapor pressure” is a more scientifically accurate term because air does not “hold” water vapor like a sponge holds water.*** Pressure gauge ***air molecules only co-exist with water vapor molecules! Pressure gauge If the water is now heated, what will happen to the evaporation rate? That’s right! The added energy will increase the evaporation rate because heat is absorbed by the liquid molecules (remember evaporation is dependent on temperature!) Pressure gauge That’s right! The added energy will increase the evaporation rate because heat is absorbed by the liquid molecules (remember evaporation is dependent on temperature!) Pressure gauge And what will happen to the vapor pressure? The vapor pressure will increase…until… Pressure gauge a new, higher equilibrium vapor pressure is reached. Pressure gauge a new, higher equilibrium vapor pressure is reached. Pressure gauge Until a new, higher equilibrium vapor pressure is reached. Pressure gauge In summary… The higher the temperature, the more moisture is required for the equilibrium vapor pressure to be reached. More water molecules are needed in the air (and returning to the liquid phase) to balance the evaporating molecules “Saturation” is a term that might make it easier to explain this phenomenon, but it is inaccurate It is not the case that warm air “holds” more water vapor, it is just that the evaporation rate is higher at this temperature, and therefore the equilibrium level is higher. If warm air is very dry (low in water vapor), it won’t reach the equilibrium level! Cold air doesn’t “hold” less water vapor, it is just that the evaporation rate decreases at lower temperatures, and therefore the equilibrium vapor pressure is lower. Colder air does not require as much moisture to reach the equilibrium level. Less water molecules are needed in the air (and returning to the liquid phase) to balance the evaporating ones Therefore, cooling air (with a fixed amount of water vapor) makes it “easier” for the equilibrium vapor pressure to be reached. …this is why cooling air favors condensation (but more about this later!) IMPORTANT TO KNOW BEFORE WE MOVE ON… Equilibrium vapor pressure is the pressure (due to temperature) at which evaporation = condensation The amount of water vapor required for equilibrium varies according to temperature For every 10o C increase in temperature, the amount of water vapor required for equilibrium (“saturation”) almost doubles! TEMPERATURE (oC) Equilibrium mass of water vapor (g) per kilogram of dry air -30 0.3 -20 0.75 -10 2 0 3.5 10 7 20 14 30 26.5 40 47 Data taken from Lutgens and Tarbuck IMPORTANT TO KNOW BEFORE WE MOVE ON… The evaporation rate is dependent on TEMPERATURE The condensation rate is dependent on the HUMIDITY The atmosphere acts much like our closed container in the model described before: Gravity, rather than a lid, prevents water vapor from escaping into space Water vapor is constantly evaporating from liquid surfaces (lakes, oceans, etc.) just like it was in our closed container However, in nature, a balance between evaporation and condensation is not always achieved… More water molecules may leave the surface of a drop of water than return to it: (NET EVAPORATION or “drying”) Liquid water molecules Water vapor molecule More water molecules may leave the surface of a drop of water than return to it: (NET EVAPORATION or “drying”) Liquid water molecules More water molecules may leave the surface of a drop of water than return to it: (NET EVAPORATION or “drying”) Water vapor molecule More water molecules may leave the surface of a drop of water than return to it: (NET EVAPORATION or “drying”) EVAPORATION (E) > CONDENSATION (C) The liquid drop gets smaller and will completely evaporate! OR…More water molecules may condense on the surface of a drop of water than leave it: (NET CONDENSATION or “wetting”) More water molecules may condense on the surface of a drop of water than leave it: (NET CONDENSATION or “wetting”) More water molecules may condense on the surface of a drop of water than leave it: (NET CONDENSATION or “wetting”) More water molecules may condense on the surface of a drop of water than leave it: (NET CONDENSATION or “wetting”) More water molecules may condense on the surface of a drop of water than leave it: (NET CONDENSATION or “wetting”) CONDENSATION (C) > EVAPORATION (E) The liquid drop gets bigger! In order for clouds to form, we need C>E! Well then, what conditions will favor condensation over evaporation? Decreasing temperatures (remember that amount of water vapor required for equilibrium is lower at lower temperatures.) High water vapor pressure (which translates to high humidity: large quantities of water vapor in the air) Condensation nuclei (a critical component we will discuss soon!) We can think of the equilibrium vapor pressure as the “turning point” because this is where E = C (a balance point.) The “scale” can be “tipped” in either direction here: EQUILIBRIUM We can think of the equilibrium vapor pressure as the “turning point” because this is where E = C (a balance point.) The “scale” can be “tipped” in either direction here: CLOUDS DRY UP! We can think of the equilibrium vapor pressure as the “turning point” because this is where E = C (a balance point.) The “scale” can be “tipped” in either direction here: Cloud droplets can grow! How can we track the evaporation rate versus the condensation rate in order to determine if clouds will form??? BY USING THE FORMULA FOR RELATIVE HUMIDITY RELATIVE = HUMIDITY Air’s water vapor content Amount of water vapor required for equilibrium vapor pressure X 100 Since the condensation rate is proportional to the air’s water vapor content we can substitute into the formula below… RELATIVE = HUMIDITY Condensation Air’s water vapor Rate content Amount of waterRate vapor Evaporation required for equilibrium vapor pressure Since the amount of water vapor required for equilibrium is proportional to the evaporation rate, we can also substitute this into the formula X 100 Relative Humidity indicates how near the air is to the equilibrium (when C=E) RELATIVE = HUMIDITY Condensation Rate Evaporation Rate When the condensation rate and the evaporation rate are equal (equilibrium), the relative humidity is 100%. X 100 Relative Humidity indicates how near the air is to the equilibrium (when C=E) RELATIVE = HUMIDITY Condensation Rate Evaporation Rate When the condensation rate is 1/2 the evaporation rate, the relative humidity is 50%. X 100 Relative Humidity indicates how near the air is to the equilibrium (when C=E) RELATIVE = HUMIDITY Condensation Rate Evaporation Rate When the condensation rate is 1/4 the evaporation rate, the relative humidity is 25%. X 100 How do we determine how close we are to a relative humidity of 100%? (cloudbuilding levels) By determining the difference between the air temperature and the DEW POINT TEMPERATURE DEW POINT TEMPERATURE is the temperature at which air would need to be cooled to reach the equilibrium vapor pressure This is the temperature at which C=E! Above this temperature, the evaporation rate will outpace the condensation rate CLOUDS DRY UP! Above this temperature, the evaporation rate will outpace the condensation rate CLOUDS DRY UP! Above this temperature, the evaporation rate will outpace the condensation rate CLOUDS DRY UP! Above this temperature, the evaporation rate will outpace the condensation rate CLOUDS DRY UP! Below the dew point temperature, the condensation rate will outpace the evaporation rate Cloud droplets can grow! This means there will be more water vapor in the air than the equilibrium level. Cloud droplets can grow! Balance will be obtained again as this “extra” water vapor condenses out to form clouds. Cloud droplets can grow! Balance will be obtained again as this “extra” water vapor condenses out to form Will decrease as clouds. condensation RELATIVE HUMIDITY = Air’s water vapor content Amount of water vapor required for equilibrium vapor pressure This is why relative humidity doesn’t usually exceed 100%! increases X 100 Dew Point Temperature, unlike Relative Humidity, measures the actual moisture content of the air. If the Dew Point Temperature is high (ex. 85o F), it means that the equilibrium vapor pressure is also high. This means that there is so much water vapor in the air, the air doesn’t need to be cooled much for relative humidity to equal 100% (equilibrium.) Even though the evaporation rate is high (high temperature), the condensation rate is also high because there is so much water vapor in the air RELATIVE = HUMIDITY Condensation Rate Evaporation Rate Does not need to be lowered much for C=E! X 100 If the Dew Point Temperature is low (ex. 33o F), it means that the equilibrium vapor pressure is also low. This means that the air is so dry (C rate so low), the air must be cooled a lot for relative humidity to equal 100% (equilibrium.) The amount of moisture in the air is so low that the evaporation rate must be lowered A LOT (by lowering temperature) for the condensation rate to equal the evaporation rate. RELATIVE = HUMIDITY Condensation Rate Evaporation Rate Must be lowered a lot for C=E! X 100 For every 10o C (18o F) increase in dew point temperature, the air contains twice as much water vapor. Dry air but high R.H. Air Temp. Dew Pt. Temp. R.H. = ?100% Moister air, but lower R.H.! Moist air AND high R.H.! How does Relative Humidity Change? #1 By the addition or removal of water vapor If water vapor is added to air this rate will increase…and R.H. will too! RELATIVE = HUMIDITY Condensation Rate X Evaporation Rate 100 How does Relative Humidity Change? #1 By the addition or removal of water vapor If water vapor is removed from air this rate will decrease…and R.H. will too! RELATIVE = HUMIDITY Condensation Rate X Evaporation Rate 100 INITIAL CONDITION 1 kg air 7 g water vapor 20o C Vapor required for equilibrium = ? TEMPERATURE (oC) Equilibrium mass of water vapor (g) per kilogram of dry air -30 0.3 -20 0.75 -10 2 0 3.5 10 7 20 14 30 26.5 40 47 Data taken from Lutgens and Tarbuck INITIAL CONDITION 1 kg air R.H. = ? 7 g water vapor 20o C Vapor required for equilibrium = 14 g 7g RELATIVE = HUMIDITY Air’s water vapor content Amount of water vapor required for equilibrium vapor pressure 14 g X 100 INITIAL CONDITION 1 kg air R.H. = 50% 7 g water vapor 20o C Vapor required for equilibrium = 14 g Addition of 5 grams of water vapor by evaporation, NO temperature change! 1 kg air 7 g water vapor + 5 g =12 g E 20o C Vapor required for equilibrium = 14 g Addition of 5 grams of water vapor by evaporation, NO temperature change! 1 kg air R.H. = 12 100 14 12 g water vapor E 20o C Vapor required for equilibrium = 14 g Addition of 5 grams of water vapor by evaporation, NO temperature change! 1 kg air R.H. = 86% **in a sealed container, we will eventually reach 100% even without heating!** 12 g water vapor E 20o C Vapor required for equilibrium = 14 g When moist air mixes with drier air, it may raise the relative humidity of the drier air to 100%. This can happen without cooling the air any further. (humidity increases and the condensation rate now exceeds the evaporation rate!) Evaporation from the pond increases humidity in the air over the pond. This creates a “cloud” (fog) over the water when there is none over the land! Fog over St. John’s Pond: Cold Spring Harbor, NY St. John’s Pond Foggy Morning at Judy’s Pond—Newcomb, NY How does Relative Humidity Change? #2 Temperature increases or decreases RELATIVE = HUMIDITY Condensation Rate X Evaporation Rate If temperature decreases, this rate will too, and R.H. will increase! 100 How does Relative Humidity Change? #2 Temperature increases or decreases RELATIVE = HUMIDITY Condensation Rate X 100 Evaporation Rate In addition, the vapor pressure will decrease. This means less water vapor is needed for equilibrium. How does Relative Humidity Change? #2 Temperature increases or decreases RELATIVE = HUMIDITY Condensation Rate X Evaporation Rate As a result, the amount of vapor present in the air may become enough for clouds to form, even if it wasn’t before (at higher temperatures.) 100 INITIAL CONDITION 1 kg air R.H. = 50% 7 g water vapor 20o C Vapor required for equilibrium = 14 g COOLING AIR TO 10o C 1 kg air 7 g water vapor 10o C Vapor required for equilibrium = ? g TEMPERATURE (oC) Equilibrium mass of water vapor (g) per kilogram of dry air -30 0.3 -20 0.75 -10 2 0 3.5 10 7 20 14 30 26.5 40 47 Data taken from Lutgens and Tarbuck COOLING AIR TO 10o C 1 kg air R.H. = ? 7 g water vapor 10o C Vapor required for equilibrium = 7 g COOLING AIR TO 10o C 1 kg air R.H. = 7 g 100 7g 7 g water vapor 10o C Vapor required for equilibrium = 7 g COOLING AIR TO 10o C 1 kg air R.H. = 100 % 7 g water vapor A change in R.H. without the addition of any water vapor! 10o C Vapor required for equilibrium = 7 g A real-life example of this is when fog and dew form early in the morning (especially on humid days)when air temperatures are at their lowest. This especially happens on and around plants because they enrich air with humidity with the transpiration from their leaves. Early morning view from the top of Mount Goodnow (Newcomb, NY) How does Relative Humidity Change? #2 Temperature increases or decreases RELATIVE = HUMIDITY Condensation Rate X Evaporation Rate If temperature increases, this rate will too, and R.H. will decrease! 100 How does Relative Humidity Change? #2 Temperature increases or decreases RELATIVE = HUMIDITY Condensation Rate X Evaporation Rate Now the vapor pressure will increase, and it will require more vapor for equilibrium. 100 How does Relative Humidity Change? #2 Temperature increases or decreases RELATIVE = HUMIDITY Condensation Rate X Evaporation Rate The amount of water vapor in the air will not be enough for the condensation rate to balance evaporation. 100 INITIAL CONDITION 1 kg air R.H. = 50% 7 g water vapor Assume no liquid water in flask 20o C Vapor required for equilibrium = 14 g HEATING AIR TO 30o C 1 kg air 7 g water vapor 30o C Vapor required for equilibrium = ? g TEMPERATURE (oC) Equilibrium mass of water vapor (g) per kilogram of dry air -30 0.3 -20 0.75 -10 2 0 3.5 10 7 20 14 30 26.5 40 47 Data taken from Lutgens and Tarbuck HEATING AIR TO 30o C 1 kg air 7 g water vapor 30o C Vapor required for equilibrium = 26.5 g HEATING AIR TO 30o C 1 kg air R.H. = ? 7 g water vapor 30o C Vapor required for equilibrium = 26.5 g HEATING AIR TO 30o C 1 kg air R.H. = 7 g 100 26.5 g 7 g water vapor 30o C Vapor required for equilibrium = 26.5 g HEATING AIR TO 30o C 1 kg air R.H. = 26% A decrease in R.H. without a change in water vapor 7 g water vapor 30o C Vapor required for equilibrium = 26.5 g Fog and dew dissipate later in the morning, once temperatures have risen above the dew point temperature View from Mt. Goodnow, several hours after the previous picture There is a physical problem to making clouds that must be discussed, as we have not yet discussed an important ingredient of cloud formation. Clouds are composed of microscopic particles of liquid water (averaging under 0.02 mm!) They must be small or they would not remain suspended in air! The rate of evaporation from a water droplet increases as the size of the molecule decreases. Liquid Water Droplet Size and Evaporation Rate EVAPORATION RATE INCREASING Cloud droplets don’t grow instantaneously, they do it gradually. This presents a problem, because for a tiny droplet to grow, it must be in an environment such that the rate of condensation is greater than that of evaporation. Liquid Water Droplet Size and Evaporation Rate EVAPORATION RATE INCREASING If a typical “baby” cloud droplet of radius 0.001 millimeter is to grow, the relative humidity of its environment must be greater than 300% because the rate of evaporation from such a small drop is so great. Relative Humidities of 300% are not observed in our atmosphere, yet clouds do exist! So something else must be needed to save these budding clouds. Cloud droplets can survive by latching onto microscopic solid particles, or condensation nuclei in our atmosphere. These solid particles can be dust, smoke, and salt particles. From volcanoes From Forest Fires The Ocean Pollution (First three pictures are not by the author) Salt water droplets from the ocean are carried by updrafts into the atmosphere. When the water evaporates, the salt is left behind. The best condensation nuclei are hygroscopic, or water absorbent We can think of them as water-droplet “magnets” Water vapor molecules Condensation Nucleus The best condensation nuclei are hygroscopic, or water absorbent We can think of them as water-droplet “magnets” The best condensation nuclei are hygroscopic, or water absorbent We can think of them as water-droplet “magnets” The best condensation nuclei are hygroscopic, or water absorbent We can think of them as water-droplet “magnets” The best condensation nuclei are hygroscopic, or water absorbent We can think of them as water-droplet “magnets” The best condensation nuclei are hygroscopic, or water absorbent We can think of them as water-droplet “magnets” The best condensation nuclei are hygroscopic, or water absorbent We can think of them as water-droplet “magnets” Liquid water (drops coalesced together) Condensation nuclei allow a water droplet to grow to a size large enough that can now avoid being dried out by evaporation. Condensation nuclei hold the liquid droplets long enough so another vapor molecule can condense on it. Condensation nuclei hold the liquid droplets long enough so another vapor molecule can condense on it. They increase the probability that more water molecules will “hit” the growing drop rather than leave it! Condensation nuclei hold the liquid droplets long enough so another vapor molecule can condense on it. They increase the probability that more water molecules will “hit” the growing drop rather than leave it! Condensation nuclei hold the liquid droplets long enough so another vapor molecule can condense on it. They increase the probability that more water molecules will “hit” the growing drop rather than leave it! Due to condensation nuclei, clouds can form even at relative humidities that are below 100%! (Even as low as 75%!) Condensation nuclei hold the liquid droplets long enough so another vapor molecule can condense on it. They increase the probability that more water molecules will “hit” the growing drop rather than leave it! Due to condensation nuclei, clouds can form even at relative humidities that are below 100%! (Even as low as 75%!) If the condensation nuclei is soluble (such as salt), they are even more effective at keeping the growing liquid droplet together. The reason for this is that dissolving anything in water lowers the vapor pressure of the water (lowers the evaporation rate!) What factors result in cloud formation? Air rising and cooling to the dew point by expansion (adiabatic cooling) By forced lifting—such as when air is forced over a mountain: Pictures from the National Audubon Society Field Guide to Weather Air rising and cooling to the dew point by expansion (adiabatic cooling) By forced lifting—such as when less dense warm air is forced above more dense cold air (when two air masses meet) Air rising and cooling to the dew point by expansion (adiabatic cooling) By forced lifting—such as when less dense warm air is forced above more dense cold air (when two air masses meet) Air rising and cooling to the dew point by expansion (adiabatic cooling) By convection: The Sun heating the ground (by radiation), which then heats the air above (by conduction), which then rises due to convection (is less dense than the cooler air surrounding it.) Picture from the National Audubon Society Field Guide to Weather Clouds can be made of ice too, and we have not introduced this idea into our discussion yet. It is part of an important process of cloud formation that occurs at temperatures around –10 to –20o C Ice-crystal cirrus clouds The equilibrium vapor pressure above ice crystals is lower than above liquid droplets. The reason for this is that ice crystals are solid, and the individual water molecules are held more tightly together than the water molecules in a liquid (greater attractive forces in the solid state.) As a result, it is easier for water molecules to escape from the liquid droplets (attractive forces are less-higher vapor pressure) Where liquid water and ice co-exist, water vapor will move from where it is higher concentration to lower concentration. Higher vapor pressure above liquid Liquid water Lower vapor pressure above ice Ice NOTE It is possible for ice and liquid water to co-exist at below freezing temperatures. Liquid water can be supercooled well below 0o C and will not freeze unless it contacts a “freezing nuclei” Where liquid water and ice co-exist, water vapor will move from where it is higher concentration to lower concentration. Higher vapor pressure above liquid Liquid water Lower vapor pressure above ice Ice The ice crystals will collect more of these liquid molecules than it will lose, because it has such a low vapor pressure. The liquid turns to ice on contact with the growing ice cloud. Higher vapor pressure above liquid Liquid water Lower vapor pressure above ice Ice As a result, the ice crystal cloud will grow at the expense of a liquid cloud nearby because of the transfer of molecules from high to low concentration. Higher vapor pressure above liquid Liquid water Lower vapor pressure above ice Ice As a result, the ice crystal cloud will grow at the expense of a liquid cloud nearby because of the transfer of molecules from high to low concentration. Higher vapor pressure above liquid Liquid water Lower vapor pressure above ice Ice As a result, the ice crystal cloud will grow at the expense of a liquid cloud nearby because of the transfer of molecules from high to low concentration. Higher vapor pressure above liquid Lower vapor pressure above ice Turned to ice Liquid water Ice As a result, the ice crystal cloud will grow at the expense of a liquid cloud nearby because of the transfer of molecules from high to low concentration. Higher vapor pressure above liquid Liquid water Lower vapor pressure above ice Ice As a result, the ice crystal cloud will grow at the expense of a liquid cloud nearby because of the transfer of molecules from high to low concentration. Higher vapor pressure above liquid Liquid water Lower vapor pressure above ice Ice As a result, the ice crystal cloud will grow at the expense of a liquid cloud nearby because of the transfer of molecules from high to low concentration. Liquid water Ice As a result, the ice crystal cloud will grow at the expense of a liquid cloud nearby because of the transfer of molecules from high to low concentration. Liquid water Ice As a result, the ice crystal cloud will grow at the expense of a liquid cloud nearby because of the transfer of molecules from high to low concentration. Liquid water Ice A REAL picture of the previous explanation! (“Hole-punch cloud”) Picture taken from the “Astronomy Picture of the Day” website http://antwrp.gsfc.nasa.gov /apod/astropix.html SEE “APOD” explanation (in speaker notes: right click and select speaker notes) Required (and enjoyable!) reading: This book was essential for completing (and understanding) this presentation REFERENCES CONSULTED Ahrens, Donald C. Meteorology Today 7th ed. Thomson Brooks Cole, 2003. Bohren, Craig F. Clouds in a Glass of Beer: Simple Experiments in Atmospheric Physics. New York: Dover Publications Inc., 2001. Link, Robert A. Earth Science Teacher Emeritus, Plainedge Middle School (personal communication) My mentor and the one who made this all make sense in the first place. Thanks forever Bob! Lutgens, Frederick K., and Edward Tarbuck. The Atmosphere: An Introduction to Meteorology. New Jersey: Prentice Hall, 2001. Wood, Elizabeth A. Science From Your Airplane Window. New York: Dover Publications Inc., 1975.