Document
advertisement

POLYCHAR 22 - Short Course
Michael Hess
DYNAMIC-MECHANICAL and
CALORIMETRIC
PROPERTIES OF POLYMERS
Thanks to Dr. Kevin Menard, University of North Texas and Perkin Elmer for some of the examples
Calorimetric Analysis of Polymers
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
•
course of polymerization
transreactions
chain scission
radiation induced degradation
thermal degradation
chain stripping leaving carbonaceous residue
curing of thermosets
...
phase behaviour (estimation of a phase diagram)
mesophase behaviour
(processing) history
(themoreversible) gelation
thermal transitions (Tg, Tm, Tcr...)
bound water in hydrogels
crystallization and ordering
...
Dynamic-Mechanical
Analysis
mechanical properties of (polymeric) materials under the influence of
dynamic load and temperature
mechanical modulus as a function of load, temperature, time
transitions, relaxations, service range (temperature), service time
Thermal Transitions
transition temperatures, transition enthalpie
glass transition
cold crystallization
•recrystallization
•crystallization
melting
•brittle/viscous
•miscibility
•thermal history
•polymorphism
•mesophases
•phase diagram
•kinetics
•degree of crystallization
•purity
DTA
DT
DSC
Wel (DT=0)
poly(dimethyl siloxane) (PDMS)
heat flux
or
cp
melting
Tm (end)
enthalpy relaxation
cold crystallization
Tg
Tm (onset)
Tm (rate max)
Dynamic-mechanical analysis - rheology
the modern machines can rapidly change the measuring device
so that solid and fluid samples can be measured
and many different modes can be applied
Thermomechanical Analysis
Stress-Strain Curves
Creep Recovery
Stress Relaxation
Dynamic Mechanical Analysis
Solvent Immersed testing
simple deformations in a solid
simple extension
simple shear deformation
(also in liquids possible)
simple stress in a shear deformation
ii = normal stress; ij = shear stress
22
21
12
32
23
2
1
3
33
31
13
11
The total stress ij is a second rank tensor composed of normal and
shear components
(ideal) energy elasticity
•caused by deformation of bond angles and bond length at small deformations
•the energy is stored and completely released after the load is removed
•there is no (internal) friction
=
Fnormal
= E
=
=
A0
1
E
=
L L0
L0
=
DL
L0
= 1
Fin plane
A0
= G
= J
= strain; = uniaxial deformation ratio; = shear (angle); F = force [N]; A0 = initial area
E = Young modulus [Pa]; G = shear modulus [Pa]; J = compliance [Pa-1]
Stress Causes Strain
Lo
elongationL-Lo = DL
L
Cauchy or
Engineering Strain
= DL/Lo
Hencky or
True Strain
= ln (DL/Lo)
Kinetic Theory
of Rubber Strain
= 1/3{L/Lo-(Lo/L)2}
Kirchhoff Strain
= 1/2{ (L/Lo)2-1}
Murnaghan Strain
= 1/2{1-(Lo/L)2}
The different definitions of tensile strain
become equivalent at very small deformations.
The stress [Pa = N/m2] refers to the initial cross section
Stress and strain are principally time-dependent
stress can “relax” (at constant strain)
elongation can “creep” (at constant stress)
ideal stress-strain diagram
inThe
the elastic limit: Hooke’s Law
=
slope = k
Strain increases
with increasing
Stress
Slope: elastic (Young-) modulus E
the major types of moduli
extension
Young modulus E
shear
shear modulus G
compression
bulk modulus B
bending*
bending modulus Eb
*three-point bending, 4 point bending
E = 2G 1 ) = 3 B 1 2 )
lat
=
long
The lateral strain lat is the strain
normal to the uniaxial deformation.
the different moduli can be converted into one another, see D. Ferry
so that for elastomers:
0.5E 3G
The volume change on deformation is for most elastomers negligible so that
=0.5 (isotropic, incompressible materials).
In a sample under small uniaxial deformation!!
The lateral strain lat is the strain
normal to the uniaxial deformation.
shear in an ideal (Newtonian) liquid
ideal liquid between
two parallel plates
an ideal liquid shows no elasticity
dilatant
grad v
= grad v =
dv
dx 2
= shear rate
=
= dynamic viscosity [Pa s]; 1 centipoise = 1 mPa s
structural
viscous
slope =
.
important rheometer types for viscous samples
torque
plate-plate
cone-plate
constant shear rate
along the radius
Couette
a combination of both:
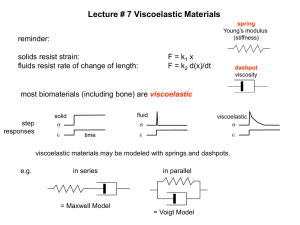
visco-elastic behaviour
James Clerk Maxwell, Phil. Trans. Roy. Soc. London 157 (1867) 52
single relaxation time
spectrum of relaxation times i
There is mater that shows elastic and viscous behaviour (e.g. pitch):
fast deformation rather elastic, slow deformation rather viscous response
major response types on deformational stress
storage and loss of energy
dissipated energy
Qrev = E '' 02
1
wrev = E
2
'
E”
2
0
Young’s modulus is
designed for elastic
materials. Real materials
consist of both elastic
and viscous response.
E” – lost to friction and
rearrangement - “the
Loss Modulus”
saved energy
E’
tan =
E ''
E'
E’ – stored and released –
“the Storage Modulus”
(conceptually like
Young’s Modulus)
damping (factor)
'simple' static stress-strain experiment
tensile strength
b
stress
= f , T )
= f t , T )
tensile strength
yield strength
brittleness B*):
B=
elongation at break
tensile strength
1
b E '
elongation at break
*) according to Brostow et al., J. Mater. Sci 21 (2006) 2422
not to be confused with the bulk modulus B (compression modulus)
strain
b
b
time
frequency dependence of damping
TTS
temperature
superposition
temperature depending properties (elasticity, flow…)
long term prediction, fatigue…
frequency range and applied technique
DMA relates:
product properties
molecular structure
Material
Behavior
processing conditions
Free damping experiment
An
=ln
A
n 1
amplitude
logarithmic decrement
shear (storage) modulus G'(f, T)
loss modulus G''(f, T)
loss factor D (tangent)
stress
strain
stress and strain are in phase in an ideal energy-elastic material,
phase angle = 0°
stress and strain are out of phase in an ideal viscous material,
phase angle = 90°
The modulus of a visco-elastic material is a complex physical entity
Theory shows that the modulus is complex and can be split into
a real part E ' and an imaginary part with E '':
0 i 0
0
0
= E * = e = cos i sin ) = cos i sin
0
0
0
0
E'
E ''
E * = E ' iE ''
1
wrev = E ' 02
2
Qrev = E '' 02
dissipated (loss)
stored
Because Young’s Modulus isn’t
enough…
E”
Young’s modulus is
designed for elastic
materials. Real materials
consist of both elastic
and viscous response.
E” – lost to friction and
rearrangement - “the
Loss Modulus”
E’
E’ – stored and released –
“the Storage Modulus”
(conceptually like
Young’s Modulus)
Correlation between moduli and phase angle (damping)
in the Gaussian plane of complex numbers the
complex shear modulus G*, the Young modulus E*
and the complex viscosity * can be visualized as
E* = ; G* = ; * =
the damping factor D is then given by the tan of
the loss angle
D= tan
D shows a behaviour similar to
Modulus, damping and their correlation with molecular motions
or tan
(rubber)
or tan
a thermodynamic view at the 'glass transition'
There is not only
one glass.
The type of glass
depends on the
thermal history.
slowly heating can cause
annealing
G'
The glass transition in a dynamic experiment
tan
G''
glassy
visco-elastic
rubbery
T
DMA and different molecular parameters
Curv e 1: DMA Temp/Time Scan in Extension
File inf o:
demof ilm
Wed Oct 11 17:06:48 1995
Frequency : 1.00 Hz
Amplitude: 21.949u
pet f ilm
Tension: 110.000%
# 1 pet f ilm:demof ilm
tan
Tg are easily seen, as in PET Film
# 2 Storage Modulus (Pa x 10
9
)
1.6
3.5
3.0
1.0
2.5
9
)
1.2
0.8
Onset 83.29
C
2.0
0.6
1.5
0.4
Onset 107.82
0.2
1.0
C
0.5
0.0
Onset 79.35
C
0.0
-100.0
0.0
100.0
Temperature (
TEMP1: -100.0 C
TEMP2: 250.0 C
TIME1: 0.0 min
RATE1: 10.0 C/min
200.0
C)
300.0
PERKIN-ELMER
7 Series Thermal
Analy sis Sy stem
Sun Nov 26 21:02:11 1995
Modulus (Pa x 10
tan
1.4
Tg by DMA and DSC
differential scanning calorimetry
Peak Tan = 140.5°C
Onset E’ = 133.1 °C
Tf
Heat flow/mW
Onset E” = 127.3 °C
Inflection Point
Onset
Onset Tan = 130.0 °C
Temperature /C
Temperature/C
(a)
(b)
DCp
DH/J/g)
Tan
Modulus/Pa
Peak E” = 136.7 °C
Operating Range by DMA
Curv e 1: DMA Temp/Time Scan in 3 Point Bending
File inf o:
gamma_1
Thu Jun 30 02:17:24 1988
Frequency : 7.00 Hz
Dy namic Stress: 1.86e+06Pa
EPOXY PC BOARD AT 7 Hz Static Stress: 1.86e+06Pa
# 1 EPOXY PC BOARD AT 7 Hz:gamma_1
10
Storage Modulus (Pa x 10
)
1.1
4.5
Tg
0.8
10
)
Operating
range
)
5.0
Beta
0.9
-1
(x 10
4.0
)
(b)
tan
3.5
2.5
Operating
range
0.5
0.4
2.0
1.5
1.0
0.2
0.5
0.1
0.0
0.0
-100.0
modulus is ok
Curv e 1: DMA Temp/Time Scan in 3 Point Bending
File inf o:
AMPf rPP.1 Wed Oct 27 13:49:06 1993
Frequency : 1.00 Hz
Dy namic Stress: 950.0mN
LeBrun samples
Static Stress: 1000.0mN
TIME1: 0.0 min
RATE1: 10.0 C/min
# 1 LeBrun samples:AMPf rPP.1
Storage Modulus (Pa x 10
tan
(x 10
-1
3.0
Operating
range
1.5
1.5
1.0
1.0
0.5
0.5
0.0
-150.0
-100.0
AMP Flame Retardant Poly propy lene
TIME1: 0.0 min
RATE1: 5.0 C/min
-50.0
0.0
Temperature (
50.0
C)
-1
leather-like state
(x 10
2.0
2.0
)
2.5
tan
9
)
200.0
PE DMA7 R&D LAB
PERKIN-ELMER
7 Series Thermal
Analy sis Sy stem
Sun Nov 26 20:13:53 1995
9
)
)
2.5
TEMP1: -160.0 C
TEMP2: 300.0 C
100.0
C)
toughness is ok
#2
Modulus (Pa x 10
0.0
Temperature (
TEMP1: -180.0 C
TEMP2: 300.0 C
100.0
KPM
PERKIN-ELMER
7 Series Thermal
Analy sis Sy stem
Sun Nov 26 20:58:36 1995
(x 10
3.0
0.6
tan
Modulus (Pa x 10
0.7
0.3
(c)
-1
(a)
-> # 2
1.0
temperature of use
shear modulus
rigid
blow
vacuum forming extrusion
'leather'
forming
injection moulding
rubber
viscous
amorphous
semicrystalline thermoplasts
shear modulus
temperature of use
extrusion
injection moulding
cold
forming
vacuum
forming
blow
forming
Cold Crystallization in PET seen by DMA and DSC
Tm
Tg
Cold Crystallization
DSC
Curv e 1: DMA Temp/Time Scan in 3 Point Bending
AMP66gp.1 Tue Oct 26 16:05:29 1993
File inf o:
Dy namic Stress: 190.0mN
Frequency : 1.00 Hz
Static Stress: 200.0mN
LeBrun samples
Higher Order Transitions affect toughness
LeBrun samples
4.0
5.5
5.0
3.5
4.5
)
-1
2.5
3.0
2.5
2.0
b Transitions
1.5
2.0
Tg
Poor
1.5
1.0
1.0
0.5
0.5
0.0
0.0
-150.0
-100.0
-50.0
AMP good part 20% glass f illed Ny lon 6/6
Impact was
good if Tg/Tb was 3 or less.
TEMP1: -160.0 C
TEMP2: 300.0 C
TIME1: 0.0 min
RATE1: 5.0 C/min
0.0
Temperature (
50.0
C)
100.0
150.0
KPM
PERKIN-ELMER
Analy sis Sy stem
7 Series Thermal
Sat Oct 15 14:32:54 1994
(x 10
Good Impact Strength
3.5
tan
Modulus (Pa x 10
9
)
3.0
4.0
stress-relaxation in a silicone rubber
DMTA
Tg
DSC
Cold Crystallization
Left: silicon rubber with a glass transition at –117°C and a melting transition at –40°C. Beyond the
melting temperature this crosslinked (vulcanised) material shows rubber-elasticity with modulus that
increases with the temperature.
Right: also a silicone rubber that contains silicone oil as diluent, as plasticizer. The oil causes a stressrelaxation at the beginning of the melting transition around –47°C.
Blends and Copolymers
Polymer A
Both Tgs
E’
E’
Block Copolymers
Graft Copolymers
Immiscible Blends
Temperature/K
Temperature/K
+
=
Single Tg
E’
E’
Polymer B
Exact T depends on
concentration of A and B
Temperature/K
Temperature/K
Random
Copolymers &
Miscible Blends
The frequency-dependence of dynamic experiments
Temperature dependency of E' and tan of PVC at different frequencies, after
Becker, Kolloid-Z.140 (1955) 1
Time-Temperature-Superposition Principle(TTS)
experimental
experimental
window
window
NBS-poly(isobutylene, after A. Tobolski
The glass transition temperature seen by viscosity
lg
empirical WLF equation
Tg+50K
Arrhenius-type
Temperature-dependence of the viscosity
of PMMA (M=63.000 g/mol) after Bueche
Williams-Landel-Ferry (WLF) equation
Ts )
20, 4 T Ts )
ln
= ln aT =
; Ts = Tg 50 ) K
)
T
102 T Ts
TTS gives the frequency-dependence
of the glass transition temperature:
lg AT
lim
T Tg T Tg
= 0.338
t
g
0.338T Tg ) = lg AT = lg = lg
t
g
g
lg
DT =
0.338
An increase of the measuring frequency (heating rate) by a factor 10 (or a decrease of
the time frame by a factor of 10) near Tg the glass-transition temperature is found about
3 K higher.
Master Curves*) extend the range
• We can collect data from 0.01 to 100 Hz.
• If we do this at many temperatures, we can
“superposition” the data.
TTS
• After TTS, our range is 1e-7 to 1e9 Hertz (1/sec)
• Then x scale (frequency) can then be inverted to get
time *) modulus or compliance; compliance = (modulus)-1
BUT...
TTS assumes that:
“all relaxation times are equally affected
by temperature.”
THIS IS KNOWN TO OFTEN BE
INVALID.
J. Dealy
Log J*
Failure of TTS
compliance J = 1/E
Analysis of a Cure by DMA
E’-E” Crossover ~ gelation point
10 8
10 7
Modulus
10 6
106 Pa ~ Solidity
10 5
vitrification point
E”
10 4
Curing
10 3
E’
10 2
Melting
Minimum Viscosity (time, length,
temperature )
10 1
10 0
50.0
70.0
90.0
110.0
130.0
150.0
T
experiment at a constant heating rate
time-temperature-transition diagram
after Gillham
Activation Energy tells us about the molecule
• For example, are these 2 Tgs or a Tg and a Tb?
Elastomer
Sample
• Because we can calculate the Eact for the peaks, we
can determine if both are glass transitions.
Determination of the apparent energy of activation
log f =
Ea 1
const .
R T
146.5 kJ/mol
351.7 kJ/mol
How can we do this experimentally??
MULTIPLEXING
Multiplexing…
Instead of just the Tg
Sheet
Film
Fiber
multiple frequencies in one run
Or you can use the Synthetic Oscillation Mode
Take five frequencies
Sum together
And
apply the
complex
wave
form to
the
sample
Temperature in C
Gelation Point by Multiplexing
We can then do further analysis
Activation energy
Master curve
PMMA
(0.01~100Hz)
Why?…
To Review, DMA ties together...
molecular structure
Molecular weight
MW Distribution
Chain Branching
Cross linking
Entanglements
Phases
Crystallinity
Free Volume
Localized motion
Relaxation Mechanisms
product properties
Material
Behavior
processing conditions
Stress
Strain
Temperature
Heat History
Frequency
Thermal
Pressure
Heat set
Dimensional Stability
Impact properties
Long term behavior
Environmental resistance
Temperature performance
Adhesion
Tack
Peel
Further Readíng
Kevin P. Menard, Dynamic-Mechanical Analysis, CRC-Press (1999) Boca Raton
W. Brostow, Performance of Plastics, Carl Hanser Verlag (2000) Munich
I. M. Ward, Mechanical Properties of Solid Polymers, Wiley (1983) New York
J. J. Aklonis, W. J. McKnight, Introduction to Polymer Viscoelasticity,
Wiley-Interscience (1983) New York
N. W. Tschloegl, The Theory of Viscoelastic Behaviour, Acad. Press (1981) New York
D. Ferry, Viscoelastic Properties of Polymers, Wiley (1980) New York
B. E. Read, G. D. Dean, The Determination of Dynamic Properties of Polymers and
Composites, Hilger (1978) Bristol
L. E. Nielsen, Polymer Rheology, Dekker (1977) New York
L. E. Nielsen, Mechanical Properties of Polymers, Dekker (1974) New York
L. E. Nielsen, Mechanical Properties of Polymers and Composites Vol. I & II,
Dekker (1974) New York
A. V. Tobolsky, Properties and Structure of Polymers, Wiley (1960) New York
Many examples by courtesy of Kevin Menard
(University of North Texas, Department of Materials Science
and Perkin Elmer Corp.)