Adsorption On Solid Surface
advertisement
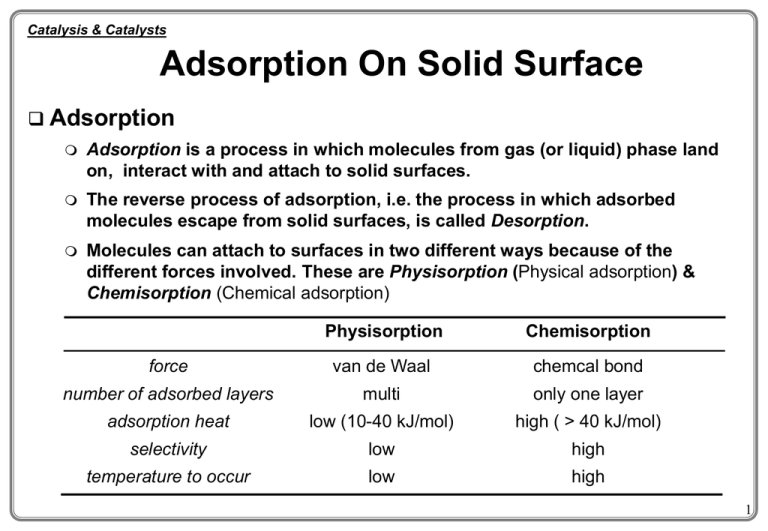
Catalysis & Catalysts Adsorption On Solid Surface Adsorption Adsorption is a process in which molecules from gas (or liquid) phase land on, interact with and attach to solid surfaces. The reverse process of adsorption, i.e. the process in which adsorbed molecules escape from solid surfaces, is called Desorption. Molecules can attach to surfaces in two different ways because of the different forces involved. These are Physisorption (Physical adsorption) & Chemisorption (Chemical adsorption) Physisorption Chemisorption force van de Waal chemcal bond number of adsorbed layers multi only one layer adsorption heat low (10-40 kJ/mol) high ( > 40 kJ/mol) selectivity low high temperature to occur low high 1 Catalysis & Catalysts Solid Catalysts Catalyst composition Active phase Where the reaction occurs (mostly metal/metal oxide) Promoter Textual promoter (e.g. Al - Fe for NH3 production) Electric or Structural modifier Poison resistant promoters Support / carrier Catalyst Support Increase mechanical strength Increase surface area (98% surface area is supplied within the porous structure) may or may not be catalytically active 2 Catalysis & Catalysts Solid Catalysts Some common solid support / carrier materials Alumina Inexpensive Surface area: 1 ~ 700 m2/g Acidic Silica Inexpensive Surface area: 100 ~ 800 m2/g Acidic Other supports Active carbon (S.A. up to 1000 m2/g) Titania (S.A. 10 ~ 50 m2/g) Zirconia (S.A. 10 ~ 100 m2/g) Magnesia (S.A. 10 m2/g) Lanthana (S.A. 10 m2/g) Active site Zeolite mixture of alumina and silica, often exchanged metal ion present shape selective acidic porous solid pore 3 Pores of Porous Solids Pore sizes micro pores dp <20-50 nm meso-pores 20nm <dp<200nm macro pores dp >200 nm Pores can be uniform (e.g. polymers) or non-uniform (most metal oxides) Pore size distribution Typical curves to characterise pore size: Cumulative curve Frequency curve Uniform size distribution (a) & non-uniform size distribution (b) dw dd wt Dwt a b Dd a b d Cumulative curve d Frequency curve 4 Catalysis & Catalysts Adsorption On Solid Surface Adsorption process Adsorbent and adsorbate Adsorbent (also called substrate) - The solid that provides surface for adsorption high surface area with proper pore structure and size distribution is essential good mechanical strength and thermal stability are necessary Adsorbate - The gas or liquid substances which are to be adsorbed on solid Surface coverage, q The solid surface may be completely or partially covered by adsorbed molecules define number of adsorption sites occupied q= number of adsorption sites available q = 0~1 Adsorption heat Adsorption is usually exothermic (in special cases dissociated adsorption can be endothermic) The heat of chemisorption is in the same order of magnitude of reaction heat; the heat of physisorption is in the same order of magnitude of condensation heat. 5 Catalysis & Catalysts Adsorption On Solid Surface Applications of adsorption process Adsorption is a very important step in solid catalysed reaction processes Adsorption in itself is a common process used in industry for various purposes Purification (removing impurities from a gas / liquid stream) De-pollution, de-colour, de-odour Solvent recovery, trace compound enrichment etc… Usually adsorption is only applied for a process dealing with small capacity The operation is usually batch type and required regeneration of saturated adsorbent Common adsorbents: molecular sieve, active carbon, silica gel, activated alumina. Physisorption is an useful technique for determining the surface area, the pore shape, pore sizes and size distribution of porous solid materials (BET surface area) 6 Adsorption On Solid Surface Characterisation of adsorption system Adsorption isotherm - most commonly used, especially to catalytic reaction system, T=const. The amount of adsorption as a function of pressure at set temperature Adsorption isobar - (usage related to industrial applications) The amount of adsorption as a function of temperature at set pressure Adsorption Isostere - (usage related to industrial applications) Adsorption pressure as a function of temperature at set volume V3>V2 V2>V1 T2 >T1 T3 >T2 T4 >T3 P3>P2 P2>P1 P1 V4>V3 V1 Pressure T1 Vol. adsorbed P4>P3 Vol. adsorbed T5 >T4 Pressure Adsorption Isotherm Temperature Adsorption Isobar Temperature Adsorption Isostere 7 Adsorption On Solid Surface The Langmuir adsorption isotherm Basic assumptions surface uniform (DHads does not vary with coverage) monolayer adsorption, and no interaction between adsorbed molecules and adsorbed molecules immobile Case I - single molecule adsorption A when adsorption is in a dynamic equilibrium A(g) + M(surface site) D AM the rate of adsorption rads = kads (1-q) P the rate of desorption rdes = kdes q at equilibrium rearrange it for q let case I rads = rdes kads (1-q) P = kdes q q (kads / kdes ) P 1 (kads / kdes ) B0 P B0 kads kdes q Cs BP 0 C 1 B0 P B0 is adsorption coefficient 8 Adsorption On Solid Surface The Langmuir adsorption isotherm (cont’d) Case II - single molecule adsorbed dissociatively on one site A-B(g) + M(surface site) D A-M-B the rate of A-B adsorption rads=kads (1-qA )(1-qB)PAB=kads (1-q )2PAB q=qA=qB the rate of A-B desorption rdes=kdesqAqB =kdesq2 at equilibrium rads = rdes rearrange it for q Let. q kads (1-q )2PAB= q2 A A B B case II kdes (k ads / k des ) PAB 1 (k ads / k des ) PAB k B0 ads kdes Cs ( B0 PAB )1/2 q C 1 ( B0 PAB )1/2 9 Adsorption On Solid Surface The Langmuir adsorption isotherm (cont’d) Case III - two molecules adsorbed on two sites A(g) + B(g) + 2M(surface site) D A-M + B-M the rate of A adsorption rads,A = kads,A (1- qA- qB) PA the rate of B adsorption rads,B = kads,B (1- qA- qB) PB the rate of A desorption rdes,A = kdes,A qA the rate of B desorption rdes,B = kdes,B qB at equilibrium rearrange it for q where rads ,A = rdes ,A A B case III and rads ,B = rdes ,B kads,A(1-qA-qB)PA=kdes,AqA and kads,B(1-qA-qB)PB=kdes,BqB qA Cs ,A B0 ,A PA C 1 B0 ,A PA B0 ,B PB B0 ,A kads ,A kads ,B and B0 ,B kdes ,A kdes ,B qB Cs ,B B0 ,B PB C 1 B0 ,A PA B0 ,B PB are adsorption coefficients of A & B. 10 Adsorption On Solid Surface The Langmuir adsorption isotherm (cont’d) A A case I C BP q s 0 C 1 B0 P k B0 ads kdes B qA Cs ( B0 PAB )1/2 q C 1 ( B0 PAB )1/2 kads kdes B0 ,A Adsorption Adsorption Strong kads>> kdes B0>>1 q Cs 1 C Weak kads<< kdes B0<<1 q A, B both strong kads>> kdes B0>>1 q case III B0 ,A PA Cs ,A C 1 B0 ,A PA B0 ,B PB C B0 ,B PB q B s ,B C 1 B0 ,A PA B0 ,B PB case II B0 A B Cs 1 C kads<< kdes Cs C B0 P B0<<1 q s ( B0 P)1/2 C C A strong, B weak A weak, B weak kads ,A k and B0 ,B ads ,B kdes ,A kdes ,B Cs ,A B0 ,A PA C B0 ,A PA B0 ,B PB C B0 ,B PB q B s ,B C B0 ,A PA B0 ,B PB qA q A C s , A / C 1 q B Cs ,B / C ( B0 ,B / B0 ,A ) q A Cs ,A / C B0 ,A PA q B Cs ,B / C B0 ,B PB PB PA 11 Adsorption On Solid Surface case I case II Case III q adsorption isotherm Cs BP 0 C 1 B0 P Cs ( B0 PAB )1/2 q C 1 ( B0 PAB )1/2 C B0 ,A PA q A s ,A C 1 B0 ,A PA B0 ,B PB C B0 ,B PB q B s ,B C 1 B0 ,A PA B0 ,B PB Strong adsorption Weak adsorption Amount adsorbed Langmuir kads>> kdes q Cs 1 kads<< kdes C C q s B0 P C mono-layer large B0 (strong adsorp.) moderate B0 small B0 (weak adsorp.) Pressure Langmuir It adsorption isotherm established a logic picture of adsorption process fits many adsorption systems but not at all The assumptions made by Langmuir do not hold in all situation, that causing error Solid surface is heterogeneous thus the heat of adsorption is not a constant at different q Physisorption of gas molecules on a solid surface can be more than one layer 12 Adsorption On Solid Surface Five types of physisorption isotherms are found over all solids I amount adsorbed II III IV V 1.0 relative pres. P/P0 Type I Type II for non-porous materials Type III porous materials with cohesive force between adsorbate molecules greater than the adhesive force between adsorbate molecules and adsorbent Type IV staged adsorption (first monolayer then build up of additional layers) Type V porous materials with cohesive force between adsorbate molecules and adsorbent being greater than that between adsorbate molecules is found for porous materials with small pores e.g. charcoal. It is clearly Langmuir monolayer type, but the other 4 are not 13 Adsorption On Solid Surface Other adsorption isotherms Many other isotherms are proposed in order to explain the observations The Temkin (or Slygin-Frumkin) isotherm Assuming the adsorption enthalpy DH decreases linearly with surface coverage From ads-des equilibrium, ads. rate des. rate rads=kads(1-q)P rdes=kdesq B0 P b1eQs / RT P q qs 1 B0 P 1 b1eQs / RT P DH of ads Langmuir Temkin where Qs is the heat of adsorption. When Qs is a linear function of qi. Qs=Q0-iS (Q0 is a constant, i is the number and S represents the surface site), the overall coverage q [b1eQs / RT P RT 1 b1P q q s dS dS ln 1 b P exp(- i 0 0 (1 b eQs / RT P i 1 1 RT 1 1 When b1P >>1 and b1Pexp(-i/RT) <<1, we have q =c1ln(c2P), where c1 & c2 are constants Valid for some adsorption systems. 14 Adsorption On Solid Surface The Freundlich isotherm assuming logarithmic change of adsorption enthalpy DH with surface coverage From ads-des equilibrium, ads. rate des. rate DH of ads rads=kads(1-q)P rdes=kdesq B0 P b1eQi / RT P q qi 1 B0 P 1 b1eQi / RT P Langmuir Freundlich q where Qi is the heat of adsorption which is a function of qi. If there are Ni types of surface sites, each can be expressed as Ni=aexp(-Q/Q0) (a and Q0 are constants), corresponding to a fractional coverage qi, i qi Ni 0 [b1eQ / RT P / (1 b1eQ / RT P)] aeQ/Q0 dQ the overall coverage q Q/Q0 N i a e dQ i 0 the solution for this integration expression at small q is: lnq=(RT/Q0)lnP+constant, or as is the Freundlich equation normally written, q c1 p1/ C where c1=constant, 1/c2=RT/Q0 2 Freundlich isotherm fits, not all, but many adsorption systems. 15 Adsorption On Solid Surface BET (Brunauer-Emmett-Teller) isotherm Many physical adsorption isotherms were found, such as the types II and III, that the adsorption does not complete the first layer (monolayer) before it continues to stack on the subsequent layer (thus the S-shape of types II and III isotherms) Basic assumptions the same assumptions as that of Langmuir but allow multi-layer adsorption the heat of ads. of additional layer equals to the latent heat of condensation based on the rate of adsorption=the rate of desorption for each layer of ads. the following BET equation was derived P / P0 1 c -1 ( P / P0 ) V ( 1 - P / P0 ) cVm cVm Where P - equilibrium pressure P0 - saturate vapour pressure of the adsorbed gas at the temperature P/P0 is called relative pressure V - volume of adsorbed gas per kg adsorbent Vm -volume of monolayer adsorbed gas per kg adsorbent c - constant associated with adsorption heat and condensation heat Note: for many adsorption systems c=exp[(H1-HL)/RT], where H1 is adsorption heat of 1st layer & HL is liquefaction heat, so that the adsorption heat can be determined from constant c. 16 Adsorption On Solid Surface Comment on the BET isotherm BET equation fits reasonably well all known adsorption isotherms observed so far (types I to V) for various types of solid, although there is fundamental defect in the theory because of the assumptions made (no interaction between adsorbed molecules, surface homogeneity and liquefaction heat for all subsequent layers being equal). BET isotherm, as well as all other isotherms, gives accurate account of adsorption isotherm only within restricted pressure range. At very low (P/P0<0.05) and high relative pressure (P/P0>0.35) it becomes less applicable. The most significant contribution of BET isotherm to the surface science is that the theory provided the first applicable means of accurate determination of the surface area of a solid (since in 1945). Many new development in relation to the theory of adsorption isotherm, most of them are accurate for a specific system under specific conditions. 17 Adsorption On Solid Surface Use of BET isotherm to determine the surface area of a solid At low relative pressure P/P0 = 0.05~0.35 it is found that P / P0 1 c -1 ( P / P0 ) ( P / P0 ) V ( 1 - P / P0 ) cVm cVm Y The = a +b P / P0 V (1- P / P0 ) P/P0 X principle of surface area determination by BET method: A plot of P / P0 V (1- P / P0 ) against P/P0 will yield a straight line with slope of equal to (c-1)/(cVm) and intersect 1/(cVm). For a given adsorption system, c and Vm are constant values, the surface area of a solid material can be determined by measuring the amount of a particular gas adsorbed on the surface with known molecular cross-section area Am, V As Am N m Am m 6.022 1023 VT , P Vm - volume of monolayer adsorbed gas molecules calculated from the plot, L VT,P - molar volume of the adsorbed gas, L/mol Am - cross-section area of a single gas molecule, m2 * In practice, measurement of BET surface area of a solid is carried out by N2 physisorption at liquid N2 temperature; for N2, Am = 16.2 x 10-20 m2 18 Adsorption On Solid Surface Summary of adsorption isotherms Name Isotherm equation Cs BP 0 C 1 B0 P Application Note Chemisorption and physisorption Useful in analysis of reaction mechanism q =c1ln(c2P) Chemisorption Chemisorption Freundlich q c1 p1/ C2 Chemisorption and physisorption Easy to fit adsorption data BET P / P0 1 c -1 ( P / P0 ) V ( 1 - P / P0 ) cVm cVm Multilayer physisorption Useful in surface area determination Langmuir q Temkin 19 The BET isotherm Theoretical development based on several assumptions: OT fig1.3 multimolecular adsorption 1st layer with fixed heat of adsorption H1 following layers with heat of adsorption constant (= latent heat of condensation) constant surface (i.e. no capillary condensation) gives p 1 C -1 p v a (p 0 - p v m C v m C p 0 or p p I s v a (p 0 - p p0 20 The BET isotherm, cont. Plot of left side vs. p/p0 should give straight line with slope s and intercept I p p I s v a (p 0 - p p0 OT fig1.5 Reorganizing gives 1 Is and C sI I Knowledge of S0 (specific area for a volume of gas then allows the calculation of the specific surface area Sg: vm Sg v m S0 mp where mp is the mass of the sample 21 BET cont’d BET method useful, but has limitations microporous materials: mono - multilayer adsorption cannot occur, (although BET surface areas are reported routinely) assumption about constant packing of N2 molecules not always correct? theoretical development dubious (recent molecular simulation studies, statistical mechanics) - value of C is indication o f the shape of the isotherm, but not necessarily related to heat of adsorption 22 Simplified method 1-point method simplefied BET assuming value of C 100 (usually the case), gives p 1 C -1 p p ' v a (p 0 - p v m C v m C p 0 v m p 0 v 'm v a (p 0 - p p0 usually choose p/p0 0,15 method underestimates the surface area by approx. 5%. 23 Adsorbates An adsorbate molecule covers an area , calculated assuming dense packing of the molecules in the multilayer. The corresponding area per volume gas is S0: Gas Temp. [K] σ [Å2/molecule] S0 [m2/cm3 gas (STP)] N2 Kr Ar H2O C2H6 CO2 77,5 77,5 77,5 298 90 195 16,2 19,5 14,6 10,8 22,5 19,5 4,36 5,24 3,92 2,90 6,05 5,24 24 Porosity and pore size The pore structure (porosity, pore diameter, pore shape) is important for the catalytic properties pore diffusion may influence rates pores may be too small for large molecules to diffuse into Measurement techniques: Hg penetration interpretation of the adsorption - desorption isotherms electron microscopy techniques 25 Hg penetration Based on measuring the volume of a non-wetting liquid forced into the pores by pressure (typically mercury) Surface tension will hinder the filling of the pores, at a given pressure an equilibrium between the force due to pressure and the surface tension is established: P r 2 -2 r cos where P = pressure of Hg, is surface tension and is the angle of wetting Common values used: = 480 dyn/cm and = 140° give average pore radius r 75000 Å 2 P[kp / cm ] valid in the range 50 - 50000Å 26 Pore size distribution If the Hg-volume is recorded as a function of pressure and this curve is differentiated we can find the pore size distribution function V(r)=dV/dr OT fig 2.3. 27 The Kelvin equation If adsorbent is mesoporous we get Type IV isotherm Deviation upwards is due to filling of mesopores by capillary condensation curved liquid meniscus in narrow _ pores with radius rk: - 2 V rk p RT ln p0 V is molar volume of the liquid, minus sign introduced since in the actual range of measurement 0 < p/p0 <1 28 The Kelvin equation Since capillary condensation is preceeded by multilayer adsorption on the wall the value is corrected with t, the thickness of this layer: Cylindrical pores: rp = rk + t Parallell sided slits: dp = rk + 2t Value of t determined from measurements without capillary condensation Practical experience, typical values give for circular pores: rk - 9,547 [ Å] p ln p0 Values for t have been found to be a function of rk, e.g. for rk > 20Å: t 0,429 (ln rk 2, 61 Å 29 Adsorption-desorption hysteresis Hysteresis is classified by IUPAC (see fig.) Traditionally desorption branch used for calculation H1: narrow distribution of mesopores H2: complex pore structure, network effects, analysis of desorption loop misleading Handbook fig 2 s 431 H2: typical for activated carbons H3 & 4: no plateau, hence no well-defined mesopore structure, analysis difficult H3: typical for clays 30