LECT 06 Glycogenregulation
advertisement
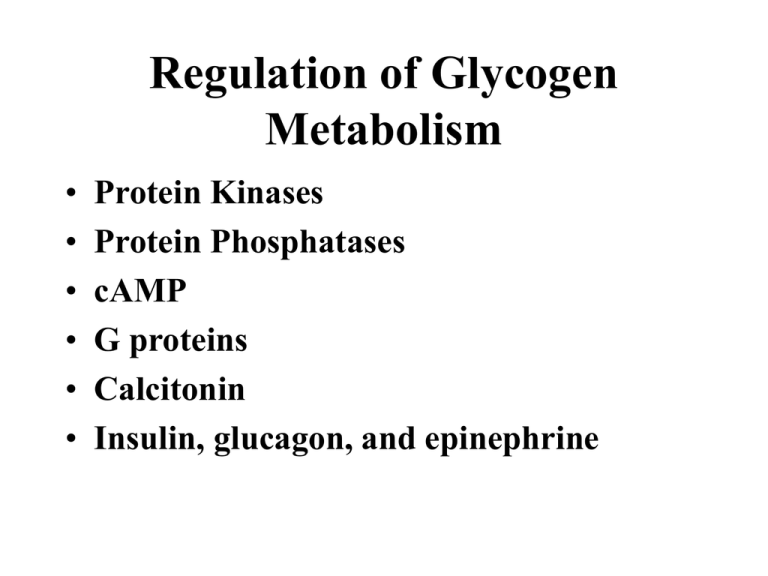
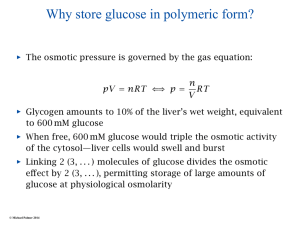
Regulation of Glycogen Metabolism • • • • • • Protein Kinases Protein Phosphatases cAMP G proteins Calcitonin Insulin, glucagon, and epinephrine Biochemical Definitions Consider A B C 1. Equilibrium: (Kinetics) When the rate of the forward reaction matches the rate of the reverse reaction. Does not specify quantity at equilibrium. 2. Equilibrium constant: (Thermodynamics) The quantity [B]/[A] at equilibrium. Does not specify rate. 3. Flux: Net carbon flow in one direction 4. Steady-state: A dynamic condition that allows flux without changing the concentration of components in the pathway. Connecting Kinetics with Thermodynamics Chemistry o ∆G = RT ln Keq Biochemistry pH = 7.0 (10-7 M [H+] ) ∆G = RT ln Keq o’ Practically Speaking….. 1. A negative ∆Go’ means [P] > [S] at equilbrium. A B ’ o ∆G = RT ln Keq [B]/[A] 2 10 100 1,000 10,000 100,000 ∆Go’ (kJ/mol) -1.8 -5.9 -11.9 -17.8 -23.7 -29.7 Steady-State vs Equilibrium Reactions Rule: An equilibrium reaction occurs in a closed system whereby two components, a reactant and a product, achieve a constancy of concentration based on chemical potentials A B Rule: A steady-state reaction occurs in an open system whereby two or more components achieve a constancy of concentration based on similar rates of components entering and leaving the system Y A B Z See Strategies p 163-167 Representing a Steady-State k1 A k2 When k1 = k2 A is constant When k 1 < k2 A decreases When k 1 > k2 A increases Basics of Metabolic Homeostasis Rule: A shift away from a dynamic steady-state evokes factors to restore the steady-state. Rule: Restoring steady-state requires modulating the activity of a rate-controlling enzyme(s) in the pathway Enzyme activity can be modulated by: 1. Covalent modification 2. Changes in pathway [S] or enzyme cofactors 3. Allosterism (Vmax or Km) 4. Hormonal intervention 5. Enzyme turnover Regulation of Carbon Flux • Net carbon flux through an individual step in a pathway is defined as the difference between the forward and reverse reaction velocities J = VF - VR (these are rates of change) • At equilibrium: VF= VR; J = 0; i.e., there is no net flux even though VF and VR could be large • When VF >> VR, J = VF • At steady state, J = k (constant) • At steady state, J depends on the rate determining (slowest) step in the pathway A B C D Rate-determining Textbook p591 Meaning of Flux Glucose J=0 VF > VR Rate-controlling Step Lactate Flux varies with equilibrium position AA A A A A BB B 1. If [A] = 100mM; [B] = 10mM, an increase in [A] by 10 mM would increase flux would by 10%. (from 10:1 to 11:1) 2. If [A] = 20mM; [B] = 10mM, an increase in [A] by 10 mM would increase flux by 33% . (2:1 to 3:1) 3. If [A] = 10mM; [B] = 10mM, an increase in [A] by 10 mM would increase flux by 100% (1:1 to 2:1). Rule: Regulators with the lowest steady-state concentration have the greatest impact on regulation AMP (0.1 mM) Adenylate Kinase ATP + X 5 0.5 ATP (5 mM) ADP (1 mM) 2ADP = AMP + ATP ATP + X~P + 4.5 0.5 ATP + AMP 2ADP 0.5 0.5 0.5 Final Talley: Before After ATP 5 mM 5 mM ADP 1 mM 1 mM AMP 0.1 mM 0.6 mM ADP 0.5 No change No change 6 times Why are there 2 enzymes in glycogen metabolism? Glycogen Glycogenase Glycogen Phosphorylase Glucose 1-PO4 How can glycogen synthesis and degradation be tuned to the needs of the cell? Only glycogen or glucose 1-PO4 concentration can affect VF or VR Stimulating or inhibiting the enzyme cannot control direction of flux Synthase Glucose 1-PO4 UDP-Glucose Because separate enzymes control synthesis and degradation, VF and VR can vary depending on the enzyme that controls the direction. This opens the way to allosteric and covalent control of glycogen cAMP as a Regulator of Glycogen ADP Phos a Syn b Pkinase Ptase Phos b PO4 Pkinase Ptase ATP PO4 ADP Syn a ATP Protein Kinase targets of cAPK cAMP Protein kinase (cAPK) Adenyl cyclase Glucagon Epinephrine Textbook p586 ATP cAMP cAMP Protein kinase (cAPK) R R C C + 4 cAMP + C P C 2ATP cAMP R cAMP cAPK Calmodulin Inactive Kinase cAMP R cAMP C 2ADP P Active Kinase Catalytic site Example would be phosphorylase b kinase Now its ready to phosphorylate phosphorylase b Inactivation of Glycogen Synthase Priming phosphate Inactive P P H2O P P B PP1 Glycogen Synthase 3Pi A ADP Casein kinase II (primer) ATP Glycogen synthase kinase 3 (GSK3) Phosphoprotein Phosphatase-1 (PP1) Phos a ADP Pkinase PP1 Phos b PO4 ATP Syn b Pkinase PP1 PO4 Syn a Phosphorylase Kinase a H2O ATP ADP cAPK PP1 PO4 ADP Phosphorylase Kinase b ATP Remember, this is the “stripper” enzyme. It takes “off” phosphate groups on proteins Insulin stimulates glycogen synthesis in liver and muscle by blocking the action of GSK3 and activating PP1 Insulin 3 ATP 3 ATP X GSK3 CKII P P P ATP Glycogen Synthase b Glycogen Synthase a Inactive 3Pi PP1 Insulin Glucagon epinephrine X Active Glucose Text p586 Glucose6-phosphate Insulin Insulin-stimulated protein kinase Phosphoprotein Phosphatase-1 (Muscle) (site 1 phosphorylation) Glycogen ADP more active P Glycogen synthesis stimulated, breakdown blocked G subunit PP1 OH K1 ATP OH Glycogen Epinephrine, glucagon K2 G subunit PP1 OH Less active ATP (site 2 phosphorylation) ADP Glycogen Breakdown stimulated, synthesis blocked cAPK K2 P 2ATP 2ADP Glycogen G subunit (site 1 and 2 phosphorylation) Text p588 P + PP1 Inhibitor P inactive Insulin regulation of GSK3 Shut down of GSK3 by PKB Text p 587 Summary of Catabolic Hormonal Effects on Glycogen • Glucagon and Epinephrine stimulate synthesis of cAMP • cAMP activates phosphorylase b kinase that converts phosphorylase b to phosphorylase a • Phosphorylase a breaks down glycogen at an accelerated rate • cAMP also inactivates PP1, prolonging the action of phosphorylase a Summary of Anabolic Effects on Glycogen • Insulin activates glycogen synthase by stimulating phosphorylation of GDK3 • GDK3 inactivates itself allowing phosphoprotein phosphatase (PP1) to remove phosphate and activate glycogen synthase • PPI also removes phosphate from phosphorylase a thereby shutting off glycogen breakdown See Tutorial on cAMP-dependent Protein Kinases available on the web Adenylcyclase cAMP dependent protein kinase R R C C Phosphorylase kinase P Phosphorylase P Liver Text p446-447 R State T State P Glucose P High Glucose Phosphoprotein phosphatase-1 Phosphorylase a Low Glucose 5’-AMP + Binds weakly to b form Phosphorylase b Active Low glucose a form (R state) P is resistant to phosphatase High glucose a form (T state) P is vulnerable to phosphatase Summary of Phosphoprotein Phosphatase-1 (Liver) 1. The Phosphatase binds to phosphorylase a (T or R form) (It does not bind to glycogen or a G protein) 2. The T form has a serine exposed to allow hydrolysis of -PO4 3. The R form of phosphorylase a -PO4 cannot be hydrolyzed 4. Glucose converts R to T form which causes hydrolysis of -PO4 and dissociate the Phosphatase. (glucose is an allosteric effector of phosphorylase in liver) 5. The liberated Phosphatase is free to activate glycogen synthase, thereby stimulating glycogen synthesis