new-ff-Benzodiazepines-
advertisement

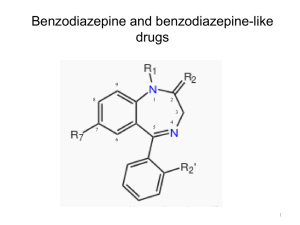
Anxiolytic sedatives (Minor tranquilizers) Anxiety is a sense of apprehensive expectation. Anxiety may be helpful e.g. anxiety before students examination but too much anxiety, however, can be deleterious due to it interferes with normal functioning Anxiolytics are used to control moderate or severe anxiety in patients with anxiety disorders. Classification I) Benzodiazepines. II) Nonbenzodiazepine agonists at BZR III) GABAA partial allosteric modulators IV) Serotonin receptor (5-HT1A) agonists I) Benzodiazepines Uses: 1. Drugs of choice for daytime anxiety and insomnia. 2. They are used as sleep inducers, selective AED (triazolam) and muscle relaxants. 3. They cause transient analgesia (I.V. diazepam). 4. Combination of benzodiazepines with CNS depressants can produce true surgical anesthesia. Benzodiazepines are ideal anxiolytics due to: Devoid of the side effects of major tranquilizers and barbiturate. High safety margin and few drug interactions than the barbiturates (not affect drug metabolism). Side effects Prolonged administration of large doses causes physical dependence and tolerance which are treated by gradual withdrawal. Mechanism of Action Anxiolytics produce their effects by enhancing the GABAnergic transmission via an increase in chloride conductance. GABA is the major inhibitory neurotransmitter in CNS. GABAA receptors are ligand-gated chloride channels. Benzodiazepines bind to benzodiazepine receptor (BZR). BZR is allosteric site and integral part of the GABAA receptor-chloride channel complex. The interaction of agonists, inverse agonists and antagonists with the BZR can be represented by the following three-state model: BZR agonists (positive modulators): Binding of benzodiazepine to BZR will increase the binding of GABA to GABAA receptor which, in turn, modulates the opening of chloride channels which may lead to anxiolytic or anticonvulsant effect. H3C CH3 O N O N N Cl O N Ph Diazepam agonist H3CO N F O H3CO CH3 Flumazenil antagonist BZR GABA receptor C2H5 CO2CH3 N N H DMCM inverse agonist BZR inverse agonists (negative modulators) bind to the GABAA receptors inducing action opposite to that of GABA due to decrease in chloride conductance that may increase the anxiety or produce convulsions. BZR antagonists (neutral modulators) occupy the GABAA receptors blocking access of agonists to the BZR e.g. flumazenil. Benzodiazepine antagonist Flumazenil (Romazicon) It is imidazobenzodiazepinone derivative. It has a high affinity to bind BZR blocking the effects of benzodiazepine e.g. sedation from benzodiazepines overdose can be reversed by flumazenil. It is ethyl 8-fluoro-5,6-dihydro5-methyl-6-oxo-4H-imidazo[1,5-a] [1,4]benzodiazepine-3-carboxylate O N O N F N CH Structure activity relationships O The following structure features should be present in benzodiazepines: Ring A An electronegative substituent at position 7 (Cl,NO2) increases activity Substituents at positions 6, 8 and 9 decrease the activity. Derivatives in which ring A (phenyl) is replaced by heterocycle show less activity. 3 CH3 Ring B The nitrogen at 1-position is essential for activity and Nsubstituent should be small whereas bulky groups sterically reduce activity. A proton-accepting group e.g.2-CO appears to be necessary to interact with receptor histadine residue that serves as a proton source. Substitution of sulfur for oxygen at the 2-position (as in quazepam) may affect selectivity for binding to receptors but activity is maintained. Alkyl substitution at 3-position reduces activity. H Compounds without 3-OH are nonpolar O 1 9 N , have long half-life and undergo hepatic 2 8 B 3 A oxidation. Compounds with 3-OH are polar N4 X 7 6 and excreted faster. 5 1` 6` 5` C 2` 3` 4` Saturation of 4, 5 double bond is not important for activity because in vivo activity results from oxidation back to C=N. Annelating the 1,2 bond of ring B with electron-rich (proton-accepting) ring such as s-triazolo (triazolam) or imidazolo(midazolam) results in active derivatives with high affinity to the BZR. Ring C A phenyl at 5-position promotes activity due to its hydrophobic interactions with receptor. If phenyl group is ortho (2’) or diortho (2’, 6’) substituted with electronattracting groups, activity is increased. Classification and Metabolism of benzodiazepines They can be divided into: A) Diazepam type. B) 3-Hydroxy and 3-carboxy benzodiazepines. C) Benzodiazepines containing F or Cl in the side chain NHCH or in ring. N D) 1,2-Annelated benzodiazepines. .HCl 3 Cl N O A) Diazepam type. Chlordiazepoxide (Librium) 7-Chloro-2-methylamino-5-phenyl-3H-1,4benzodiazepine-4-oxide. HCl It is well absorbed from GIT and its half-life is 6-30 hours (long-acting). It undergoes N-demethylation and hydrolysis of amidine to give active metabolite demoxepam. The N-oxide of demoxepam is reduced to another active metabolite, nordazepam (N-desmethyl diazepam) which is also the active metabolite for diazepam. It is hydroxylated at C3 to the active metabolite oxazepam which is rapidly glucuronated and excreted in the urine. Uses: anxiolytic, sedative, anticonvulsant and skeletal muscle relaxant. Librax = Librium + clidinium brmide (anticholinergic agent) for GIT disorders such as peptic ulcer and irritable colon. H H O N O N Diazepam Halazepam OH N Cl N Cl Clorazepate Nordazepam (active) H Oxazepam (active) H O N O N O Chlordiazepoxide Cl N Cl O HO N O OH HOOC Demoxepam (active) OH Oxazepam glucuronid CH3 Diazepam (Valium) 7-Chloro-1,3-dihydro-1-methyl-5-phenyl2H-1,4-benzodiazepin-2-one O N Cl N It is very nonpolar and rapidly absorbed with a half-life of about 20-50 hours (long-acting). The metabolism is by N-demethylation to the active nordazepam that undergoes metabolism to active metabolite oxazepam Assay: Non-aqueous titration with 0.1M perchloric acid. Uses: It is widely used as anti-anxiety, hypnotic, sedative, anticonvulsant and premedication in anesthesiology. B) 3-Hydroxy and 3-carboxy benzodiazepines Oxazepam (Serax) Oxazepam is prototype for the 3-hydroxy compounds. It is active metabolite of diazepam which much more polar than diazepam and so, marketed separately as short-acting anxiolytic agent. Lorazepam (Ativan) It is the ´2-chloro analog of oxazepam and has a short half-life (26 hours). H O N H O OH N Cl N OH Cl N Cl Lorazepam (Ativan) 7-Chloro-1,3-dihydro-3-hydroxy-5phenyl-2H-1,4-benzodiazepin-2-one 7-chloro-5-(2-chlorophenyl)1,3-dihydro-3-hydroxy-2H-1,4benzodiazepin-2-one. Clorazepate dipotassium (Tranxene) H O N 7-Chloro-2,3-dihydro-2-oxo-5phenyl-1H-1,4-benzodiazepine-3carboxylic acid dipotassium salt monohydrate. - COO K Cl N + . KOH It is polar, inactive prodrug. It is quickly converted in the GI tract to a nonpolar nordazepam via 3-decarboxylation. Nordazepam has a long half-life and then undergoes hepatic conversion to oxazepam. C) The inclusion of halogen, (C1 or F) on the aromatic ring or basic side chain seems to increase the activity and emphasize the anticonvulsant and hypnotic activity of this class of drugs. Flurazepam HCl (Dalmane) 7-chloro-1-[2-(diethylamino)ethyl] -5-(2-fluorophenyl)-1, 3-dihydro-2H1, 4-benzodiazpin-2-one dihydrochloride. Et N CF3 Et CH3 . 2HClO O N N O N N Cl O2N N Cl N F F Flurazepam Flunitrazepam It is water soluble agent and its major metabolite is N1-dealkyl flurazepam, which has a very long half-life and persists for several days. Flunitrazepam (Rohypenol) 5-(2-fluorophenyl)-1,3-dihydro-1-methyl-7-nitro -2H-1,4-benzodiazepin-2-one D) 1,2-Annelated benzodiazepines. Fused triazolobenzodiazepines Examples: alprazolam; Xanax triazolam; Halcion 8-chloro-6-(o-substitutedphenyl)-1methyl-4H-s-triazolo[4,3-a][1,4] benzodiazepine H 3C N N N Cl N X X = H : Alprazolam X = Cl:Triazolam They are metabolized by hydroxylation of the methyl group on the triazolo ring to the methyl alcohol or by 3-hydroxylation of the benzodiazepine ring. The resulting OH compound is active but is quickly conjugated and excreted and so, the duration of action is short. It is used as a sedative-hypnotic drug and not impair daytime function. II) Nonbenzodiazepine agonists at BZR Imidazopyridines derivatives They are nonbenzodiazepine compounds having affinity for BZR and show in vivo activity. R2 N O N Zolpidem R1 = R2 = R3 = CH3 Alpidem R1 = R2 = Cl, R3 = (CH2)CH3 N R1 R3 R3 Zolpidem (Ambien) N,N-Dimethyl-2-(6-methyl-2-p-tolylimidazo[1,2-a]pyridin-3-yl)acetamide hemitartrate It is a hypnotic and a relatively selective positive modulator at the benzodiazepine binding site. It is a relatively fast in action and produce no active metabolites. Alpidem It was withdrawn from the market following reports of hepatic dysfunction. III) GABAA partial allosteric modulators N CONH2 N It offers some advantages over full agonists: Partial agonists seem to have lesser side N F effects such as sedation and also Br less abuse potential. Imidazenil It is an imidazobenzodiazepine carboxamide that is more potent than diazepam. When it is given with diazepam, it blocks the sedative effects of diazepam. IV) Serotonin receptor (5-HT1A) agonists O N . HCl Buspiron, (Buspar): N N N O N It acts as anxiolytic without causing sedation. It acts as a partial agonist of serotonin at 5-HT1A receptor suppressing serotoninergic activity. It also has antidopaminergic activity, Physiochemical properties Most benzodiazepines have relatively high lipid ⁄ water partition coefficients and are completely absorbed from GIT. The 3-OH polar compounds tend to be slowly absorbed than nonpolar ones. The benzodiazepines tend to be highly bound to plasma proteins; the more nonpolar the drug, the greater the binding. They are also very effectively distributed to the brain. Compounds without 3-OH group have long half-lives and undergo conversion to the 3-hydroxyl compounds by hepatic oxidation. Compounds with 3-OH (oxazepam, lorazepam) are short-acting because they are rapidly conjugated and urinary excreted. So, they can be used in elderly or hepatic patients. Chronic administration of long-acting benzodiazepines(chlordiazepoxide,diazepam, chlorazepate,flurazepam) leads to toxic effects e.g. excessive sedation. This is due to accumulation of active metabolite nordazepam in blood for more than one week after discontinuation of the drug. The major factors considered when selecting an agent include the rate of absorption and presence or absence of active metabolites.