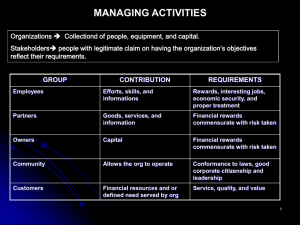
Chapter 4
advertisement

Chapter 4 Electrochemical kinetics at electrode / solution interface and electrochemical overpotential Effect of potential on electrode reaction 1. Thermodynamic aspect If electrode reaction is fast and electrochemical equilibrium remains, i.e., Nernst equation is applicable. Different potential corresponds to different surface concentration. 2. Kinetic aspect If electrode reaction is slow and electrochemical equilibrium is broken. Different potential corresponds to different activation energy. 4.1 Effect of potential on activation energy 4.1.1 basic concepts For Elementary unimolecular process A kf kb B rf k f c A Rate expressions rb kb cB rnet rf rb k f c A kb cB rnet 0; At equilibrium kb (cB ) eq k f (c A ) eq K kf kb (cB ) eq (c A ) eq Exchange rate of reaction Some important empirical formula: Arrhenius equation k Ae Ea RT According to Transition State Theory: G kT RT k e h Corresponding to steric factor in SCT For electrode reactions For reversible state Ox ne kf kb Red 0 C RT E E o ' ln o0 Nernst equation nF CR For irreversible state a b log i Tafel equation (1905) Overpotential How to explain these empirical formula? Potential curve described by Morse empirical equation Ox ne kf kb Red Standard free energy Activated complex Gc Reactant re G Ga product Reaction coordinate In electrochemistry, electrochemical potential was used instead of chemical potential (Gibbs free energy) 4.1.2 net current and exchange current ia Cu Fe3+ Cu2+ ic ic ia Fe2+ Ox ne kc ka Red ic rc kc COx (0, t ) nFA ia ra ka CRed (0, t ) nFA ic nFAkc COx (0, t ) Net current: ia nFAka CRed (0, t ) Net current: i ic ia nFA[kc cOx (0, t ) ka cRed (0, t )] i ic ia nFA[kc cOx (0, t ) ka cRed (0, t )] If cOx = cRed = activity = 1 at re At equilibrium condition Then i net = 0 ic ia i0 standard exchange current kc k a Gc kT RT kc e h Ga kT RT ka e h 4.1.3 effect of overpotential on activation energy Ox Red Na+ + e Na(Hg)x Ox Na(Hg)x Na+ + e Red Na+ + e Na(Hg)x The energy level of species in solution keeps unchanged while that of the species on electrode changes with electrode potential. polarization ir re ΔG nFE nFΔ Ox ne ΔG ΔG0 nFΔ Red ΔGc,0 c ΔG F Δ F Δ a ΔG ΔGa,0 F Δ transfer coefficient F Δ F Δ 1 Fraction of applied potential alters activation energy for oxidation and for reduction c , re nFΔ nFΔ nFΔ c ,ir Anode side cathode side a ,ir Ga Ga,0 nFΔ Gc Gc,0 nFΔ 0 tan FE / x nFΔ x nFΔ tan (1 ) FE / x tan tan tan is usually approximate to 1/2 4.1.4 Effect of polarization on reaction rate Marcus theory: transition state theory Gc ,0 nF k BT kc c exp( ) h RT G k BT nF c ,0 c exp( ) exp( ) h RT RT nF kc ,0 exp( ) RT Ga,0 nF k BT nF ka a exp( ) ka ,0 exp( ) h RT RT ic kc cOx (0, t ) nFcOx (0, t )kc,0 exp( nF RT ) No concentration polarization ic ic,0 exp( nF If initial potential is 0, then RT ) ia ia,0 exp( nF RT ) 2.3RT 2.3RT lg ic,0 lg ic nF nF 2.3RT 2.3RT lg ia,0 lg ia nF nF 2.3RT 2.3RT c re lg ic,0 lg ic nF nF 2.3RT 2.3RT a re lg ia,0 lg ia nF nF At equilibirum ia ,0 ic ,0 i0 2.3RT ic c lg nF i0 2.3RT ia a lg nF i0 lg ic 2.3RT 2.3RT c lg i0 lg ic nF nF 0 re lg i lg i0 lg ia a 2.3RT 2.3RT lg i0 lg ia nF nF 4.2 Electrochemical polarization 4.2.1 Master equation ic nFcOx (0, t )kc ,0 exp( ia nFcRed (0, t )ka,0 exp( nF RT nF RT ) ) inet ic ia nFcOx (0, t )kc ,0 exp( nF ) nFcRed (0, t )ka ,0 exp( nF RT RT nF nF nFk0 cOx (0, t ) exp( ) cRed (0, t ) exp( ) RT RT Master equation ) Theoretical deduction of Nernst equation from Mater equation inet nF nF nFk0 cOx (0, t )exp( ) cRed (0, t )exp( ) RT RT At equilibrium inet 0 cOx (0, t ) c ; 0 Ox c exp( 0 Ox nF RT )c 0 cOx nF exp( ) 0 RT cRed cRed (0, t ) c 0 Red 0 Red exp( nF RT ) 0 RT cOx ln 0 nF cRed 0 RT cOx ln 0 nF cRed Nernst equation 4.2.2 Butler-Volmer model and equation inet nF nF nFk0 cOx (0, t )exp( ) cRed (0, t )exp( ) RT RT nF nF i i0 exp( ) exp( ) RT RT Butler-Volmer equation nF nF i i0 exp( ) exp( ) RT RT 4.2.3 discussion of B-V equation 1) Limiting behavior at small overpotentials exp( nF RT ) 1 nF RT i nF nF i i0 1 1 RT RT nF nF nF i i0 1 1 i0 RT RT RT Current is a linear function of overpotential i Charge transfer resistance nF RT i i0 i/A nF Rct RTi0 Cathode Net current /V False resistance Anode 2) Limiting behavior at large overpotentials nF nF i i0 exp( ) exp( ) RT RT i/A Cathode Net current /V One term dominates exp( nF Anode ) nF RT exp 1% nF RT exp( ) RT Error is less than 1% 118 mV When cathodic polarization is larger than 118 mV inet ic ia ic i i0 exp( nF RT ) Taking logarithm of the equation gives: lg i lg i0 nF 2.3RT 2.3RT 2.3RT lg i0 lg i nF nF Making comparison with Tafel equation One can obtain 2.3RT a lg i0 nF a b lg i 2.3RT b nF At 25 oC, when n = 1, = 0.5 2.3RT b nF b 118 mV The typical Tafel slope lg i 118 mV 118 mV lg i0 300 200 100 0 -100 -200 -300 / mV Tafel plot: log i plot log i0 re 4.2.4 determination of kinetic parameters 2.3RT a lg i0 nF 2.3RT b nF For evolution of hydrogen on Hg electrode 1.40 0.118lg i 0.5 i0 1.6 1012 A cm2 i0 nFcOx (0, t )kc,0 exp( nF RT ) k 5 1013 cm s 1 active dissolution lgi i i active dissolution: n n Ag+ /Ag 0.5 0.5 Hg2+ /Hg 0.6 1.4 Cu2+ /Cu 0.4 1.6 Zn2+ /Zn 0.47 1.47 ia nFAka CRed (0, t ) ic nFAkc COx (0, t ) Gc Ga kT RT kc e h Ox ne kT RT ka e h Red ΔGc,0 ΔGc F Δ F Δ a ΔG ΔGa,0 F Δ transfer coefficient F Δ F Δ 1 c , re nFΔ nFΔ nFΔ a ,ir c ,ir Anode side cathode side Ga Ga,0 nFΔ Gc Gc,0 nFΔ ic nFcOx (0, t )kc ,0 exp( nF RT ) ia nFcRed (0, t )ka,0 exp( nF RT ) Master equation inet nF nF nFk0 cOx (0, t )exp( ) cRed (0, t )exp( ) RT RT Nernst equation 0 RT cOx ln 0 nF cRed Butler-Volmer equation nF nF i i0 exp( ) exp( ) RT RT Rct nF 2.3RT 2.3RT lg i0 lg i Tafel equation RTi0 nF nF 4.2.5 Exchange current density 1) The exchange current of different electrodes differs a lot Electrode materials solutions Hg 0.5 M sulfuric acid Cu 1.0 M CuSO4 Pt 0.1 M sulfuric acid Hg 110-3 M Hg2(NO3)2 + 2.0M HClO4 Electrode reaction i0 / Acm-2 H++2e– = H2 510-13 Cu2++2e– = Cu 210-5 H++2e– = H2 110-3 Hg22++2e– = 2Hg 510-1 2) Dependence of exchange currents on electrolyte concentration Electrode reaction c (ZnSO4) i0 / Acm-2 1.0 80.0 0.1 27.6 0.05 14.0 0.025 7.0 Zn2++2e– = Zn High electrolyte concentration is need for electrode to achieve high exchange current. Use of Ag/AgCl electrode. 2.3RT ic c lg nF i0 nF i RTi0 2.3RT ic c lg nF i0 When i0 is large and i << i0, c is small. When i0 = , c=0, ideal nonpolarizable electrode, basic characteristic of reference electrode. When i0 is small, c is large. When i0 = 0, c = , ideal polarizable electrode The common current density used for electrochemical study ranges between 10-6 ~ 1 Acm-2. If exchange current of the electrode i0 > 10~100 Acm-2, it is difficult for the electrode to be polarized. When i0 < 10-8 Acm-2, the electrode will always undergoes sever polarization. For electrode with high exchange current, passing current will affect the equilibrium a little, therefore, the electrode potential is stable, which is suitable for reference electrode. Influence of impurity If an impurity undergoes reduction at electrode * I Red I Red IOx If If I0 I Red * * I0 I Red The influence of impurity on equilibrium is negligible. * I Red IOx IRed Oxidation of electrode and reduction of impurity take place. There is net electrochemical reaction. Single/couple electrode and Mixed potential / mV lg i Icorro Electrode with exchange current less than 10-4 A cm-2 is hard to attain equilibrium potential. 4.3 Diffusion on electrode kinetic When we discuss situations in 4.2, diffusion polarization is not take into consideration. When diffusion take effect : G0.c kBT nF ic nFACOx (0, t )k exp( )exp( ) h RT RT COx (0, t ) 0 k BT G0.c nF ic nFA COx k exp( ) exp( ) 0 h RT RT COx inet COx (0, t ) nF ic i0 exp( c ) 0 RT COx inet COx (0, t ) nF ic i0 exp( c ) 0 RT COx At high cathodic polarization i nF i c ) C C (1 ) i (1 )i0 exp( id RT id s i 0 i id i i nF ( ) exp( c ) i0 id RT Therefore: id i i nF ln ln( )( c ) i0 id RT id RT i RT c ln ln nF io nF id i Electrochemical term Diffusion term The total polarization comprises of tow terms: electrochemical term and diffusion term. Discussion : id RT i RT c ln ln nF i0 nF id i 1. id >> i >> i0 No diffusion ec polarization At small polarization : i At large polarization: i c nF i i0 RT 0 c RT i c ln nF i0 id RT i RT c ln ln nF i0 nF id i 2. id i << i0 RT i c ln 0 nF i diffusion No ec is invalid id RT d ln( ) nF id i i id i log i lg i 118 mV 118 mV lg i0 300 200 100 0 -100 -200 -300 / mV id RT i RT c ln ln nF i0 nF id i 3. id i >> i0 both terms take effect 4. i << i0, id no polarization (ideal unpolarizable electrode) When id >>i0, diffusion control diff id 1 id 2 ec 1/2 re 1/ 2 1 At half wave potential i id 2 id RT i RT c ln ln nF i0 nF id i 1 id id RT RT RT 2 ln ln 2 ln nF i0 nF nF i0 RT RT ln id ln i0 nF nF d1/ 2 RT d ln i nF 0 id The half wave potential depends on both id and i0 diff id 1 id 2 id RT i RT c ln ln nF i0 nF id i ec 1/ 2 1/ 2 1/ 2 ec lgi0 diff lgid id RT ln nF i0 lg i 118 mV 118 mV lg i0 300 200 100 0 -100 -200 -300 / mV Tafel plot without diffusion polarization lg id lg i 118 mV lg i0 400 300 200 100 0 100 200 300 400 Tafel plot under diffusion polarization / mV Tafel plot with diffusion control: i0 << i < 0.1 id Electrochemical polarization i between 0.1id 0.9id mixed control i >0.9 id diffusion control Question: How to overcome mixed / diffusion control? please summarize the ways to elevate limiting diffusion current 4.4 EC methods under EC-diff mixed control 4.4.1 potential step Using B-V equation with consideration of diffusion polarization cOx (0, t ) cRed (0, t ) nF nF it i0 exp( ) exp( ) 0 0 RT RT cRed cOx at high polarization c cOx (0, t ) nF it i0 exp( c ) 0 RT cOx At constant c, it cOx(0,t) at low polarization : exp( nF RT ) (1 nF RT nF RT c is very small ) cOx (0, t ) cRed (0, t ) nF nF it i0 exp( ) exp( ) 0 0 RT RT cRed cOx cR (0, t ) cO (0, t ) cR (0, t ) nF cO (0, t ) it i0 0 0 0 0 RT cR cO cR cO Constant for potential step Numerical solution: 1 2 it i exp( 2t )erfc(t ) K c* K a* 1/ 2 1/ 2 DOx DRed i is the current density at no concentration polarization at 0 c (0, t ) c That is Ox Ox ; 0 cRed (0, t ) cRed 1 At t = 0 1 2 exp( 2t )erfc(t ) 1 0.5 1 i(0)= i no concentration polarization 2 3 2 t When 1 2 t 1 it t it ic it i t ic Double-layer charge EC control Extrapolating the linear part to y axes can obtain i c 1 2 1/ 2 1/ 2 at time right after the potential step : it t1/2 is linear 2 diff control C t1 / 2 Making potential jump to different can obtain i at different . Then plot i against c can obtain i~c without concentration polarization. The way can be used to eliminate concentration polarization. it c time constant s i Double-layer charge EC control it > i due to charge of double layer capacitor diff control C t1 / 2 i 4.4.2 current step cathodic current : 0 ic nF ic nFk ' COx (0.t )exp RT 1 2 t ci (0, t ) ci0 1 ic 0 t Record c at different ic t 1 2 0 ic nFk ' COx [1 ( ) ]exp( nF RT ) 0 nFk ' COx RT RT t 12 c (t ) ln ln[1 ( ) ] nF ic nF constant transition time when potential steps to next reaction. c t 1 2 c (t ) [1 ( ) ] 0 nFk ' COx RT RT t 12 c (t ) ln ln[1 ( ) ] nF ic nF c c(0) t 0 i= icharge c (t ) c t The slope of the linear par of c (t) can be used to determine n and . 1 2 ln[1 ( ) ] When t0 the second term = 0 c(0) ( t 0) 0 t 1 2 ln[1 ( ) ] 0 nFk ' COx RT ln nF ic 4.4.3 cyclic voltammetry (CV) (t ) 0 vt c (t ) (t ) 0 COx (0, t ) CRed (0, t ) nF nF it i0 exp( c (t )) exp( c (t )) 0 0 RT RT CRed COx for reversible single electrode I iPc Potential separation 59 Δ mV n c P iPa a P for the reversible systems , use the forward kinetics only : i nFACO (0, t )k f (t ) can be solved only by numerical method: 1 2 Ox 1 2 i nFAC D ( 0 Ox nF RT 1 2 1 2 ) x(bt ) for fast EC reaction : i << i0 controlled by diffusion Nicholson-Shain equation tramper coefficient i n – number of electrons involved in charge transfer step 1 2 x (bt ) v is tabulated x (bt) max =0.4958 0.2 0.1 0.0 0.1 0.2 For irreversible single electrode i I Pc I Pa 59 Δ mV n For totally irreversible systems RT k nFv Ep E ( )[0.78 ln ln ] F RT D peak potential shift with scan rate v i 0.2 0.1 0.0 0.1 0.2 for slow EC reaction : ii0 ( quasi reversible, irreversible) in comparison to the same rate, equilibrium can not establish rapidly. Because current takes more time to respond to the applied voltage, Ep shift with scan rate . Dependence of p on 1/ 2 D Ox DRe d 1/ 2 nF k / DOx v RT 29 kv 1/ 2 4.5 effect of 1 potential on EC rate : 1=0, validate at high concentration or larger polarization 1 G = nF x effect of 1: 1. on concentration 2. = 1 zi F C C exp( 1 ) RT i 0 i ( n zO ) F nF ic nFAk C exp( )exp( 1) RT RT 0 Ox ia nFAk 0 Re d 0 Ox 0 Re d C ( n zO ) F nF exp( )exp( 1) RT RT This means 1 has same effect on the forward (reduction) and reverse (oxidation) reaction. zO n RT i ic ia const ln i 1 nF n zO n RT c const ln i 1 nF n zO n RT c const ln i 1 nF n zO n 1 0 n When zO <0 ( minus ), n zO is large, therefore, for anion reduced on cathode , 1 effect is more significant. When zO n 1 made c shift positively so: if 1 increases, i decreases z0 n RT c const ln i 1 nF n if: n = zO = 0.5 Cu2+ +2e- = Cu MnO4 +e = MnO42 H+ +e- = 1/2 H2 RT c const ln i 1 nF if :zO = 0 RT c const ln i 1 nF adsorption of anion slow reaction without specific adsorption reduction of +1 cation …… reduction of 1 anion RT c const ln i 1 nF 1 accelerates reduction of cation, slows reduction of anion Rotation rate of RDE on reduction of 110-3 mol/L K2S2O8 without supporting electrolyte Effect of potential of zero charge on polarization curve of RDE for reduction of K2S2O8 without supporting electrolyte Only when the electrode potential is near to the potential of zero charge, 1 has large effect on the reaction rate, while at higher polarization, 1 take less effect. Effect of concentration of supporting electrolyte (sodium sulfate) on the polarization curve of RDE for reduction of K2S2O8 . 1: 0; 2: 2.8 10-3; 3: 0.1; 4: 1.0 mol/L Na2SO4 Problem: how to eliminate the effect of 1? 4.6 EC kinetics for multi-electron process For a di-electron reaction Ox + 2e Red Its mechanism can be described by ia0 Ox 1e X+1e At stable state ib0 X Red d[X] 0 dt a F a F cx i 0 ia exp( c ) 0 exp( c ) 2 RT RT cx b F a F i 0 cx ib 0 exp( c ) exp( c ) 2 RT RT cx Ox 1e X+1e ( a b ) F ( a b ) F exp c exp c i RT RT 1 2 b F 1 a F exp c 0 exp c 0 ia RT ib RT 0 0 i i If a b (1 b ) F a F i 2i exp( c ) exp( c ) RT RT 0 b ia0 ib0 X Red (1 b ) F a F i 2i exp( c ) exp( c ) RT RT 0 b Therefore i 2i 0 0 b 1 b 2 b 2 For a multi-electron reaction Ox + ne Red Its mechanism can be described by Ox 1e X1 +1e ia0 ib0 X j 1 +1e X j +1e ib0 ib0 ib0 X j 1 X j (rds) Steps before rds, with higher i0 at equilibrium ( j j 1) F ( n j) F i ni 0j exp( c ) exp( j c ) RT RT X j 1 ib0 ib0 X n 2 +1e X n 1 +1e X2 X j 2 +1e X1 X n 1 Red Steps after rds, with higher i0 at equilibrium ( j j 1) F ( j n j) F i ni exp( c ) exp( c ) RT RT 0 j Therefore i ni 0 0 j At small overpotential j j 1 n F in i c RT 2 0 j i0 n i 2 0 j j n j n At higher overpotential For cathodic current ( j j 1) ic ni exp c RT 0 j For anodic current ( j n 1) ia ni exp a RT 0 j 4.7 Marcus theory for electron transfer Effect of reactant, solvents, electrode materials and adsorbed species on electrochemical reaction. Electron-transfer between two coordination compounds. M Outer-sphere reaction M inner-sphere reaction No strong interaction between electrode surface and reactant. reactant, intermediate and product interact with electrode surface strongly. Reduction of Ru(NH3)63+ Reduction of O and oxidation of H Microscopic theories of electron transfer Electron transfer reaction, a radiationless electronic rearrangement, sharing commonalities with radiationless deactivation of excited molecules. For a homogeneous redox reaction : O + R’ R + O’ Electron transfer between tow isoenergetic points ---isoenergetic electron transfer activation Franck-Condon principle: Nuclear coordinates do not change on time scale of electronic transitions. Reactants and products share common nuclear configuration at moment of transfer. Deduce expression for standard Gibbs energy of activation as a function of structural parameters of reactant, so as to calculate rate constant of the reaction. Transition state isoenergetic electron transfer g: global reaction coordinate for 1 dimensional process, related to solvation. For homogeneous electron transfer Work of assemblying reactants, i.e., ion pair + electrostatic work to bring charged species next to charged electrode, wO and wR not considered. Improved model Predictions from Marcus theory ½ factor seems like first order term in expansion of , rest are corrections Classical Butler-Volmer theory regards as constant, cannot predict potential dependence of . Electron transfer occurs between empty levels of electrode (or species in solution) and filled levels of species in solution (or electrode) of the same energy. For reduction - energy of occupied level of electrode must match energy level of empty state of species in solution. For oxidation - energy of empty level of electrode must match energy level of occupied state of species in solution. Energy levels of metal and species in solution form a continuum Overall rate must be evaluated by summing or integrating over all energy matched pairs. Since filled electrode states overlap with (empty) O states, reduction can proceed. Since the (filled) R states overlap only with filled electrode states, oxidation is blocked. Number of electronic states of electrode in energy range E and E + dE is A ( E )dE area of the electrode density of states Total number of states of electrode in given energy range E2 A ( E )dE E1 At absolute zero, energy of highest filled state is called Fermi level, At higher temperatures, thermal energy promotes electrons to higher levels Electron distribution given by Fermi function f(E) E EF f ( E ) 1 exp kT 1 concentration density function DR (, E) Number concentration of R species in the range between E + dE is DR (, E)dE Rate Constant for Reduction Rate Constant for Oxidation FURTHER CONSIDERATIONS • Electron transfer occurs almost entirely at the Fermi level • Rate constant proportional to local rate at Fermi level. • Integrals reduce to single value R tunneling P