1 or 0
advertisement
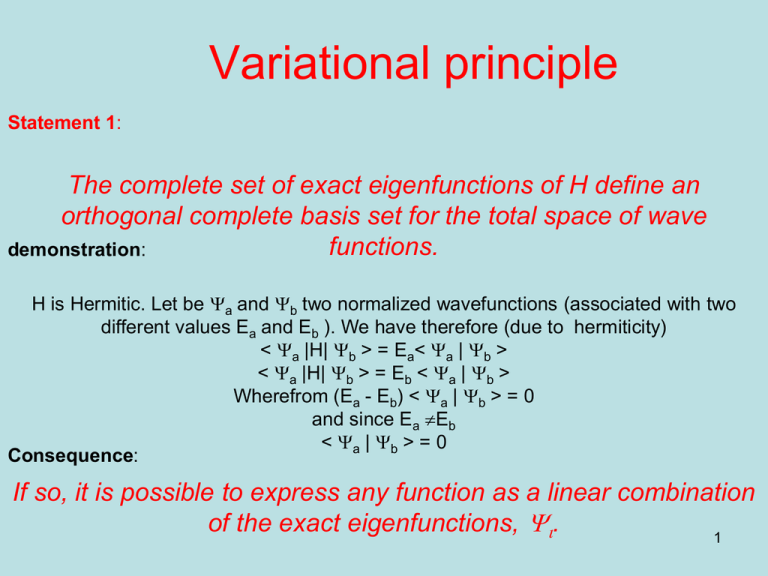
Variational principle Statement 1: The complete set of exact eigenfunctions of H define an orthogonal complete basis set for the total space of wave functions. demonstration: H is Hermitic. Let be Ya and Yb two normalized wavefunctions (associated with two different values Ea and Eb ). We have therefore (due to hermiticity) < Ya |H| Yb > = Ea< Ya | Yb > < Ya |H| Yb > = Eb < Ya | Yb > Wherefrom (Ea - Eb) < Ya | Yb > = 0 and since Ea Eb < Ya | Yb > = 0 Consequence: If so, it is possible to express any function as a linear combination of the exact eigenfunctions, Yi. 1 Variational principle Statement 2: The energy associated with Y a function is always above that of the eigenfunction of lowest energy: E0. Y is not a eigenfunction; it is associated with an energy <E> that is an average energy (mean value) A mean value is always intermediate relative to extreme: Greater than the smallest! <E> > E0 2 Mean value • If Y1 and Y2 are associated with the same eigenvalue o: O(aY1 +bY2)=o(aY1 +bY2) • If not O(aY1 +bY2)=o1(aY1 )+o2(bY2) we define <O> = (a2o1+b2o2)/(a2+b2) Dirac notations 3 Variational principle Statement 2: The energy associated with Y a function is always above that of the eigenfunction of lowest energy: E0. Ya and Yb are two eigenfunctions < Ya |H| = < Ya | Ea and |H| Yb > = Eb | Yb> wherefrom < Ya |H| Yb > = Ea dab From statement 1, Y is a linear combination of Ya s | Ya >= Sa ca| Ya > let multiply the left by < Ya |; this leads to only one term ca= < Ya |Y > and similarly c*a= < Y |Ya > then | Y >= Sa < Ya |Y > | Ya > 4 Variational principle Statement 2, normalization: Ya and Yb are two eigenfunctions associated to Ea and Eb From statement 1, Y is a linear combination of Ya s | Y >= Sa ca| Ya > then < Y | Y > =Sa,b ca < Ya |Yb> cb < Y | Y > = Sa, ca 2 < Ya |Ya> = Sa, ca 2 = 1 5 Variational principle Statement 2, Demonstration: < Y |H | Y > = Sa,b ca < Ya |H | Yb > c b < Y |H | Y > = Sa,b Ea < Ya | Yb > c b < Y |H | Y > = Sa,b Ea ca dab c b < Y |H | Y > = Sa,Ea ca 2 E > E0 > Sa,E0 ca 2 = E0 An non-exact solution has always a higher energy than the lowest exact solution 6 Using Dirac notation Ya and Yb are two eigenfunctions < Ya |H| = < Ya | Ea and |H| Yb > = Eb | Yb > wherefrom < Ya |H| Yb > = Ea da From statement 1, Y is a linear combination of Ya s | Ya >= Sa ca| Ya > Let multiplie the left by < Ya |; this leads to only one term ca= < Ya |Y > and similarly c*a= < Y |Ya > then < Y | Y > = Sa,b ca < Ya |Yb> cb < Y | Y > = Sa,b < Ya |Y > < Ya |Yb> < Yb|Y > = Sa,b < Ya |Y > dab < Yb|Y > < Y | Y > = Sa, |< Ya |Y >| 2 =1 Demonstration < Y |H | Y > = Sa,b < Y | Ya > < Ya |H | Yb > < Y b |Y > < Y |H | Y > = Sa,b Ea < Y | Ya > < Ya | Yb > < Y b |Y > < Y |H | Y > = Sa,b Ea < Y | Ya > dab < Y b |Y > < Y |H | Y > = Sa,Ea |< Ya |Y >| 2 < Y |H | Y > > > Sa,E0|< Ya |Y >| 2 = E0 E0 7 Variation principle Given an approximate expression that depends on parameters, we must determine the parameters to minimize the energy. Within LCAO, an MO is a linear combination of AOs: We have then to chose the coefficients so to minimize the energy. Note that the variational principle is not restricted to cases of linear combinations. 8 A mean value is always higher than the lowest value ! c 32 c 22 c 12 c 02 9 Slater exponent for He F= where Z* is a parameter <E> = - Z*2/2 u.a. <T> = + Z*2/2 u.a. <V> = <F(1,Z*)|-Z*/r|F(1,Z*)> using <F(1,Z*)|1/r|F(1,Z*)> = Z* u.a. Next slide <V> = -Z*<F(1,Z*)|1/r|F(1,Z*)> = -Z*2u.a. 10 <F(1,Z*)|1/r|F(1,Z*)> = Z* u.a. demonstration 11 Slater exponent for He F= where Z* is a parameter 12 Slater exponent for He F= where Z* is a parameter Search for the energy minimum 13 LCAO Y is a linear combination of N fa s | Y >= Sa ca| fa > Atomic orbitals an approximate function Worse description than Y Using parameters: ca There are N coeffcients to determine Let minimize E with respect to each of them Therefore, there is a set of N equations, dE/dc = 0 This seems fine; however there is a problem; Which is the problem? 14 Lagrangian There is a constraint due to normalization: the N coefficients are not independent. We search the minimum of a function L of N+1 variables Which “makes no difference with H” L = <Y | Y > - E [<Y | Y > - 1 ] There is a set of linear expressions to derive dL/dc = 0 Joseph Louis de Lagrange French 1736 - 1831 Should be 0 15 Secular determinant Solving | Hij-ESij| =0, we find the Eis. This is a N degree equation of E to be solved. From N AOs, we find N energies and N MOs. We find simultaneously E and Y (eigenfunction and eigenvalues). 16 LCAO Assuming no change in the AOs *, the MOs correspond to a unitary change of the basis set of AOs. MOs are orthogonal and normalized. Sum over r atoms for a given i orbital Neglecting overlaps, Src2ir=1 for a MO, Yi and Sic2ir=1 for a AO, fi ; the AOs are distributed among the MOs Sum over i orbitals for a given r atom * This is not the case for self consistent methods. 17 Schrödinger equation for LCAO HY = EY Erwin Rudolf Josef Alexander Schrödinger Austrian 1887 –1961 H SiciFi = E SiciFi Let multiply on the left by Fj, integrate and develop Sici< |H | Fi > = E Sici < Fj |Fi > Sici (Hij-E Sij ) = 0 This is the set of linear equation solved for | Hij-E S ij |= 0 secular determinant The complete set of MOs are solution (not only the lowest). Our first approach from the variational principle emphasized the solution of lowest energy Every extreme (derivative=0) is a physical solution! 18 Hückel Theory Erich Armand Arthur Joseph Hückel German 1896-1980 Never awarded the Nobel prize! Also known for DebyeHückel theory of electrolytic solutions In the 1930's a theory was devised by Hückel to treat the p electrons of conjugated systems such as aromatic hydrocarbon systems, benzene and naphthalene. Only p electron MO's are included because these determine the general properties of these molecules and the s electrons are ignored. This is referred to as sigma-pi separability. The extended Hückel Theory introduced by Lipscomb and Hoffmann (1962) applies to all the electrons. 19 Hückel Theory We consider only 2pZ orbitals Two parameters: The atomic 2p energy level: E(2pZ) = a (~ -11.4 eV) The resonance integral for adjacent 2p orbitals Hij(C=C) = b (~ -3 eV) Erich Armand Arthur Joseph Hückel German 1896-1980 Never awarded the Nobel prize! Also known for DebyeHückel theory of electrolytic solutions E = a + x b -x = (a – E)/ b We chose defining b as negative Sij = dij (the overlap is neglected). 20 SLATER KOSTER Slater Koster for metal atoms : 9 orbitals s, p et d Several overlaps ss, sps, sds, pps, ppp, pds, pdp, dds, ddp, ddd. Phys. Rev B 94 (1954) 1498 21 EHT Theory • Only valence orbitals are described • AOs are Slater orbitals – Parameters are the Hii (atomic energy levels) – The Slater exponents • The Overlap, Sij, are rigorously calculated from the geometry and the AOs • The resonance integrals, Hij, are derived from the Sij. Wolfsberg-Helmoltz formula 22 Hij = 1.75 (Hii+Hjj)/2 Sij Polyenes For linear and cyclic systems (with n atoms), general solutions exist. Linear: Cyclic: For linear polyenes the energy gap is given as: Charles Alfred Coulson (1910-1974), English 23 Extension to heteroatoms parameter Coulombic Integral Resonance Integral Carbon aC = a bCC = b Oxygen 1 p electron aO = a + b bCO = b parameter Coulombic Integral Resonance Integral Sulfur with 2 p electrons aS = a + 0,5b bCS = 0,4b aS = a + 0,2b bCS = 0,6b Oxygen 2 p aO = a + electrons 2b bCO = 0,8b Sulfur with 1 p electron Nitrogen 1 p electron aN = a + 0,5b bCN = b Fluorine aF = a + 3b bCF = 0,7b Nitrogen 2 p electrons aN = a + 1,5b bCN = 0,8b Chlorine aCl = a + 2b bCCl = 0,4b Sulfur 2 p electrons aS = a + 0,5b bCS = 0,4b Bromine aBr = a + 1,5b bCBr = 0,3b Sulfur 1 p electron aS = a + 0,2b bCS = 0,6b CH3 group hyperconju gation aMe = a + 2b bCMe = 0,7b 24 | Hij-E Sij |= 0 f1 f2 f3 f4 f5 f6 … f1 -x 1 or 0 1 or 0 1 or 0 1 or 0 1 or 0 f2 1 or 0 -x 1 or 0 1 or 0 1 or 0 1 or 0 f3 1 or 0 1 or 0 -x 1 or 0 1 or 0 1 or 0 f4 1 or 0 1 or 0 1 or 0 -x 1 or 0 1 or 0 f5 1 or 0 1 or 0 1 or 0 1 or 0 -x 1 or 0 f6 : : 1 or 0 1 or 0 1 or 0 1 or 0 1 or 0 -x 1 means that the atoms 2 and 4 are connected, The determinant is symmetric relative to the diagonal =0 25 | Hij-E Sij |= 0 f1 f2 f3 f4 f5 f6 … f1 -x 1 or 0 1 or 0 1 or 0 1 or 0 1 or 0 f2 1 or 0 -x 1 or 0 1 or 0 1 or 0 1 or 0 f3 1 or 0 1 or 0 -x 1 or 0 1 or 0 1 or 0 f4 1 or 0 1 or 0 1 or 0 -x 1 or 0 1 or 0 f5 1 or 0 1 or 0 1 or 0 1 or 0 -x 1 or 0 f6 : : 1 or 0 1 or 0 1 or 0 1 or 0 1 or 0 -x =0 This is an N degree equation of x 26 | Ei-x |= 0 solving is a diagonalization problem y4 y6 … y1 y2 y3 y5 y1 E1-x 0 0 0 0 0 y2 0 E2-x 0 0 0 0 y3 0 0 E3-x 0 0 0 y4 0 0 0 E4-x 0 0 y5 0 0 0 0 E5-x 0 y6 : : 0 0 0 0 0 E6-x =0 A set of first degree equations: E=Ei associated with Yi 27 The diagonalization is a unitary transformation OMs are orthogonal thus Srcricrj = dij If i=j, the sum of the coefficient’s squares for a given atom is 1 (normalization) If i≠j, the sum of the products of coefficients is 0 (orthogonality) The matrix of coefficients is unitary wherefrom = Sicricsj = dij If i=j, the sum of the coefficient’s squares for a given orbital is 1 AOs are distributed among all the MOs If i≠j, the sum of the products of coefficients is 0 If all the orbitals were filled, there would be no interaction: all the r-s bond indices should be zero. 28 C2 H 4 2 e in 2 orbitals as in H2 We have solved the problem using symmetry and without solving the secular equation. f1 f2 f1 -x 1 f2 1 -x =0 Inputs are what is in the secular determinant : a b and connectivity Outputs are orbitals, energies, total energies, charges and bond indices 29 C2H4 without overlap f1 f2 f1 -x 1 f2 1 -x =0 Pu Bonding Pg antibonding Energy x=1 x=-1 coefficients -c1 + c2 = 0 c1 = c 2 c1 + c 2 = 0 c1 = -c2 0 0 -x c1 + c2 = 0 C=1/√2 Charge on atoms: 1 – Si ni cri2 Bonding lrs= Si ni cricsi N-1 linear equations; the last one is redundant. 1 for pu2 0 for pupg -1 for pu2 30 C2H4 including overlap 4e repulsion E2 = (b -ES)2 E = ± (b -ES) f2 f1 -E b -ES f2 b -ES -E =0 Pu Bonding Pg antibonding Energy b/ (1+S) -b/ (1-S) coefficients c1 = c 2 = c c = 1 /√(2+2S) 0 c1 = -c2 = c c = 1 /√(2+2S) 0 Mulliken 1 f1 Charge on atoms: – Si ni (cri2 –Ss cri csi ) Bonding OPrs= Si ni cricsiSrs Overlap populations S/(1+S) for pu2 S/(2+2S)- S/(2-2S) = -4S2/(4-4S2) ~ - S2 for pupg 31 2 OA interaction modifying a -b2/ D -D/2 D/2 b 2/ D The geometric mean of b and D/2 f1 f2 f1 D/2 -E b f2 b -D/2 -E =0 (D/2 -E) (-D/2 -E) = b2 E2 - (D2 /4) = b2 E2 = b2 + (D2 /4) E = ±√[b2 + (D2 /4)] •If D = 0, E+ = b •If b <<D, E+ = (D/2) (1 + 4 b2/ D2) 0.5 E+ = (D/2) (1 + 2 b2/ D2) = D/2 (1 + 2 b2/ D) E+ - E = b2/ D 2nd order Perturbation term 32 C2 C1 C4 Butadiene C3 C1 C2 C3 C4 The topology is C1 bond C2, C2 bond to C3 and C3 bond to C4 A linear model contains the information with more symmetry (the topology does not distinguish between cis and trans) f1 f2 f3 f4 f1 -x 1 0 0 f2 1 -x 1 0 f3 0 1 -x 1 f4 0 0 1 -x =0 33 Conservation of Orbital Symmetry H C Longuet-Higgins E W Abrahamson The Molecular orbitals are solution of the symmetry operators of the molecule. MOs from different symmetry groups do not mix. Hugh Christopher Longuet-Higgins 1923-2004 34 Butadiene S or A using symmetry for the topology C1 S 0 0 A C2 C3 C4 35 Butadiene S Symmetric C1 =0 C2 C3 C4 Mind that symmetry 1 is for all the atoms Not the reduced part x2 - x - 1 = 0 x = (1 ± √5)/2 Golden numbers 1.618 and -0.618 Coefficients - (1 ± √5)/2 c1 + c2 = 0 and normalization c12 + c22 = ½ c = 0.3717 and 0.6015 1.618 -0.618 36 olden ratio Golden ratio conjugate F 37 Golden ratio Golden ratio conjugate a= b(1+√5) a= √(b2+(2b)2) =b(1+√5) b 2b 38 Polyclete and Durer 39 x2 = 1+x The medial right triangle of this "golden" pyramid (see diagram), with sides is interesting in its own right, demonstrating via the Pythagorean theorem the relationship or 40 Fibonacci recursion, irrational numbers (incommensurable) Rabbit population, assuming that: In the "zeroth" month, there is one pair of rabbit In the first month, the first pair begets another pair In the second month, both pairs of rabbits have another pair, and the first pair dies. In the third month, the second pair and the new two pairs have a total of three new pairs, 41 and the older second pair. Spirale constructions, in nature, in music.. Related ? The Doctrine of the Mean (中庸, py Zhōngyōng) is one of the Four Books, part of the Confucian canonical scriptures. 42 Pentagon, icosahedra =1.618 F=1/ =0.618 2 43 Butadiene Symmetric S C1 C2 C3 C4 Recipe to build the same determinant Express along the AOs and gather interactions with equivalent atoms Mind that symmetry reduction makes the normalization incomplete. 44 Butadiene Antisymmetric A C1 C2 C3 C4 Mind that symmetry 1 is for all the atoms Not the reduced part x2 + x - 1 = 0 x = - (1 ± √5)/2 Golden numbers 0.618 and -1.618 Coefficients (1 ± √5)/2 c1 + c2 = 0 and normalization c12 + c22 = ½ c = 0.3717 and 0.6015 0.618 -1.618 45 Butadiene Antisymmetric A C1 C2 C3 C4 Recipe to build the same determinant Express along the AOs and gather interactions with equivalent atoms Mind that symmetry reduction makes the normalization incomplete. 46 Butadiene Small 0.3717 Large 0.6015 Large 0.6015 Small 0.3717 Large 0.6015 Small 0.3717 The number of nodes increases, the amplitude is large at the middle for the extreme; it is large on the edges for the Frontier orbitals. 47 Butadiene -.3717 -.6015 -1.618 0.3717 0.6015 0.6015 -.3717 -.618 0.6015 -.3717 -.6015 0.3717 0.618 -.3717 0.6015 0.6015 1.618 0.6015 0.3717 48 Butadiene Bonding orders Ground state 0.894 0.894 0.447 No initial statement that distinguishes 1-2 from 2-3 49 Butadiene Bonding orders Ground state 1.35Ả C=C – C=C 1.35Ả 1.46Ả No initial statement that distinguishes 1-2 from 2-3 50 Matches the Lewis formula and indicates the stabilization due to delocalization Butadiene Bonding orders Excited state C–C=C–C 0.447 0.447 0.724 51 It is no use to iterate The Hückel method is powerful since predictive. It is possible to make the parameterization dependent on the differences in bond lengths choosing b12 = b34 larger than b23 or modifying a according to the charge. This does not make the calculation predictive. It is possible to make iterations (w technique) to make the results “self consistent”; this is not recommended. This does not allow controlling the parameters. 52 Annulenes f1 f2 f3 f1 -x 1 0 f2 1 -x f3 0 ... ... fN-1 fN-1 0 0 1 1 0 0 0 1 -x 1 0 0 0 0 1 -x 1 0 fN-1 0 0 0 1 -x 1 fN 1 0 0 0 1 -x =0 Secular equation: b cr-1 -E cr + b cr+1 = 0 53 Delocalization 54 Annulenes solutions for CN Rq (fr) = fr+s An annulene is a periodic system; angle q = 2ps/N The new coefficient for r is the old one multiplied by a constant Solutions for annulenes are those of any periodic system: crystals (Bloch sums) 55 annulenes The system allows solutions Rjq with angle (qj=2jp/N). There is N rotations possible including identity (j=0 ou N) : the « simple » rotation q and the « multiple» rotations jq Functions satisfying to rotations jq=2jp/N: We build linear combinations of AOs,(Scrfr) satisfying rotation qj (Scrfr) = e-ijqj (Scrfr) with cr (j) = N-1/2 eijrqj = e –i2pjr/N . There are N solutions, with a quantum number: j. . i is the square root of -1; i2=-1 56 Rotation by 120° = 2p 2/6 (j=2, orbital y2) N exp(i2p 4/6) r=2 N r=3 N r=1 C5 x exp(-ijqj)= N exp(i2p 4/6) N exp(i2p 10/6) x exp(-i2p 2/6) = r=0 exp(i2p 2/6) r=1 r=5 N exp(i2p 2/6) 0 r=4 r=0 N N exp(i2p 8/6) 0 r=2 N exp(i2p 8/6) r=5 r=4 N r=3 N exp(i2p 10/6) N exp(i2p 10/6) The 5th AO takes the place of the first C5 x exp(i2p 2/6) 57 annulenes They are the LCAO functions satisfying the rotations qj Yj= Scrfr There are j solutions; the eigenvalues are e-ijsq 58 Benzene annulenes Two real solutions and 4 complexes Degenerate by pairs + + The complexe solutions are associated with rotations only; they are not eigenfunctions of the other symmetry operators and must be the degenerate 59 annulenes Real solutions Satisfying two mirror symmetry j = 3, AS j = ± 2, AA j = ± 2, SS j ± 1, SA j = ± 1, AS j = 0, SS 60 annulenes Orbitals from symmetry only j = 3, AS j = ± 2, AA All the coefficients are equal c=1/2 All the coefficients are equal c=1/√6 j = ± 2, SS E = 4*(< 1/2f2IHI1/2f3>+…) =b j = ± 1, AS j ± 1, SA j = 0, SS E=< 1/√6f1+.. .IHI1/√6f1+…> = 12*(< 1/√6f1fIHI1/√6f2>+…) = 61 2b annulenes Orbitals from symmetry only j = 3, AS The total density in E orbital must respect symmetry j = ± 2, AA j = ± 2, SS Degenerate orbitals: energy must be b j = ± 1, AS j ± 1, SA 1/22+c2=2/6 → c= 1/√12 02+c2=2/6 → c= 1/√3 j = 0, SS Normalization is satisfied 4*1/12+2*1/3=1 62 For any annulene of large size … the number of OMs remains in the gap from 2b to -2b -2 b -b 2b b 2b j=3 j=±2 j=±1 j=0 63 annulenes Chosing another set of planes; rotation by 60° j = 3, AS Combining orbitals YS’A’ = ½ [YSA + √3 YAS ] YA’S’ = ½ [YSA - √3 YAS ] New symmetry appears While old ones desappears j = ± 2, AA j = ± 2, SS j ± 1, SA j = ± 1, AS j = 0, SS 64 Hückel determinant for Cyclobutadiene Notations: x = ↔ E = a + x b units b (b <0) , origin a f1 f2 f3 f4 f1 -x f2 1 f3 0 f4 1 1 -x 1 0 0 1 -x 1 1 0 1 -x = 0 Two possibilities of using mirror symmetries 65 First set 1/2 1/2 1/2 1/2 66 Second set 1/2 1/√2 1/√2 1/2 67 The Jahn-Teller Theorem 1937 "any non-linear molecular system in a degenerate electronic state will be unstable and will undergo distortion to form a system of lower symmetry and lower energy thereby removing the degeneracy" There is a symmetry reduction (a geometry distortion) when the a set of HOMOs is not completely filled. Edward Teller 1908-2003 Hungarian-American 68 Hermann Arthur Jahn 1907-1979 English In an octahedral crystal field, the t2g orbitals occur at lower energy than the eg orbitals. The t2g are directed between bond axes while the eg point along bond axes. 4e-8e 3e-5e-9e 0-6e 2e-8e 3e-9e The full octahedral symmetry takes place only when the t2g set is fully occupied. 69 Cyclobutadiene: C4H4 C4H4 is unstable, it exists stabilized by asymmetric ligand (one eg orbital is stabilized). It dimerizes easily. Chain of Vn-benzenen+1: The HOMO gap vanishes for n=4; then there is a rotation of the successive benzene loosing the full D5h symmetry. V V V If the rings are eclipsed, there is no gap; the rotation of ring opens a gap. 70 B5 Boron compounds 71 Jahn-Teller effects are grouped into two categories. The first arises from incomplete shells of degenerate orbitals. It includes the first-order Jahn-Teller effect and the pseudo Jahn-Teller effect. The second arises from filled and empty molecular orbitals that are close in energy and is the second-order Jahn-Teller effect. The two categories have quite different physical bases. As a result, geometric distortions produced by the first are quite small and normally lead to dynamic effects only. In favorable cases, the second-order Jahn-Teller effect produces very large distortions, including complete dissociation of a molecule. This can occur even when the relevant molecular orbitals are separated in energy by as much as 4 eV. 72 The Jahn-Teller Theorem 1937 CuCl2 Cu2+ 9e NiCl2 Ni2+ 8e The shielding effect this has on the electrons is used to explain why the Jahn-Teller effect is generally only important for odd number occupancy of the eg level. The effect of Jahn-Teller distortions is best documented for Cu(II) complexes (with 3 electrons in the eg level) where the result is that most complexes are found to have elongation along the z-axis. 73 Apparent exceptions to the theorem are probably examples of what has been called the "dynamic Jahn-Teller effect". In these cases either the time frame of the measurement does not allow the distortion to be seen because of the molecule randomly undergoing movement or else the distortion is so small as to be negligible. For one of the copper complexes, the bond lengths are apparently identical. If the X-ray structure of the sample is redone at varying temperatures it is sometimes possible to "freeze" a molecule into a static position showing the distortions. 74 Alternant conjugated hydrocarbons Definition: Atoms of conjugated molecules could be divided into two sets of atoms (starred and unstarred atoms) so that a member of one set is formally bonded only to members of the other set. Many compounds are alternant; they are not when their structure contains an odd-member ring. 75 Alternant conjugated hydrocarbons -E cr + Ss b cs = 0 if Yj = Sr* cr fr + Ss° cs fs is an eigenfunction Y‘j = Sr* cr fr - Ss° cs fs is another one with Ej'=-Ej •Orbital energy levels are symmetric relative to a level. •If there is an odd number of orbital, one of them is nonbonding 76 Alternant conjugated hydrocarbons Non bonding orbital, SOMO The nonbonding orbital has non-zero coefficient only at the starred atoms, and the sum of the coefficient of the starred atoms attached to a given unstarred atom must equal zero. The secular equation -E cr + becomes Ss cs = 0 with E=0. Applied on a starred atoms Ss cs = 0 it gives again that cs = 0 Applied on a unstarred atoms Sr* cr = 0 it tells that the unstarred atoms belong to nodal planes. It is easy to find the coefficients from there. 77 Allyle radical The SOMO is localized on the terminal atoms species): Allyle anion The charge is -1/2 on each terminal atom H2C CH2 Allyle cation The charge is +1/2 on each terminal atom a = 1/√2 a = -1/√2 78 Dewar method for estimating aromaticity Two bonds close the cycle One bond makes the polyene If the SOMO is symmetric, it is better to make 2 bonds: S or A S S 2n+1 A 2n-1 If the SOMO is Single antisymmetric, it is carbon better to make only one bond: Polyene radical A a S S S -a 4n+2 electrons aromatic 79 4n electrons antiaromatic Dewar method for estimating aromaticity Two bonds close the cycle One bond makes the polyene -a -b pyrène -b a b a -2a -a 3a 5a -3a 0 b -b -b b There is an energy gain by forming a new cycle 80 The net charge of alternant hydrocarbons is zero. The electron density on r is 1 | coeff2 of occupied MOs| = | coeff2 of unoccupied MOs | wherefrom Sini cri2=1 demonstration : Socc cr,occ2 + Snb cr,nb2 + Svac cr,vacc2 =1 2 Socc cr,occ2 + Snb cr,nb2 = 1 Sini cr,i2 = 1 81 The bond indices between atoms of the same set is zero. (first order perturbation between atoms of the same set is zero). OMs are orthogonal thus Srcricrj = dij If i=j, the sum of the coefficient’s squares for a given atom is 1 (normalization) If i≠j, the sum of the products of coefficients is 0 (orthogonality) The matrix of coefficients is unitary wherefrom = Sicricsj = dij If i=j, the sum of the coefficient’s squares for a given orbital is 1 If i≠j, the sum of the products of coefficients is 0 Socc cr,occcs,occ + Snb cr,nbcs,nb + vac cr,vacccs,vacc =drs 2 Socc cr,occcs,occ + Snb cr,nbcs,nb = 0 Socc cr,occcs,occ + Snb cr,nbcs,nb = 0 Srnicricsj = 0 82 Butadiene is an alternant -.3717 -.6015 -1.618 0.3717 0.6015 0.6015 -.3717 -.618 0.6015 -.3717 -.6015 0.3717 0.618 -.3717 0.6015 0.6015 1.618 0.6015 0.3717 83 Benzene is alternant j = 3, AS E = -2 b annulen es j = ± 2, AA j = ± 2, SS E = -1b j ± 1, SA j = ± 1, AS E = 1b j = 0, SS E = 2b 84 Importance of the non bonding orbital • for radical species, it represents the unpaired electron •For anions or cations it monitors the charge d = Sini cr,i2 d = 2 Socc cr,occ2 + 2 cr,nb2 d = (2 Socc cr,occ2 + 1 cr,nb2 ) + cr,nb2 d = 1 + cr,nb2 For cations d = 2 Socc cr,occ2 d = (2 Socc cr,occ2 + 1 cr,nb2 ) - cr,nb2 d = 1 - cr,nb2 For anions 85 Heteroatoms:CO parameter Coulombic Integral Resonance Integral Carbon aC = a bCC = b Oxygen 1 p electron aO = a + b bCO = b Oxygen 2 p aO = a + electrons 2b fC fO fC -x 1 fO 1 -x+1 =0 x2-x-1=0 O more electronegative x= (1±√5)/2 Antibonding, large amplitude on C -0.618 bCO = 0,8b 0 0.8506 -0.5257 1 p electron for CO 0.5257 0.8506 1 Bonding, large amplitude on O C 1.618 O 86 allyle 1 2 3 1 -x 1 0 x3-2x=0 Solutions: x=- √2 x=0 x= √2 2 1 -x 1 3 0 1 -x Sym 1 2 1 -x 2 AntiSym 1 Sym 1/√2(f1+f3) 1/√2(f1+f3) -x f2 √2 2 1 -x 1 -x f2 √2 -x 87 Allyle coefficients x=0 -xc1+c2=0 c1-xc2+c3=0 c2-xc3=0 c2=0 c3=-c1 this orbital is found antisymm c2=0 no new information x=√2 -√2 c1+c2=0 c1-√2 c2+c3=0 c2= √2 c1 c3 = c1 this orbital is found symm c2-xc3=0 1/√2 c3= 1/√2 c1 = c2 These expressions have be normalized 88 1/2 -1/√2 1/2 -√2 -1/√2 1/√2 0 1/2 1/√2 1/2 Charges are controlled by the filling of the nonbonding MO. They appear on the terminal atoms. The sum of charges is the global charge √2 radical cation q1 1-2(1/2)2-1(1/√2)2 = 0 1-2(1/2)2 q2 1-2(1/√2)2 = 0 = 1/2 1-2(1/√2)2 =0 anion 1-2(1/2)2-2(1/√2)2 = -1/2 1-2(1/√2)2 = 0 89 1/2 -1/√2 1/2 -√2 -1/√2 1/√2 bond indices are independent from the filling of the nonbonding MO. 0 1/2 1/√2 1/2 √2 radical l12=l23 2(1/2)(1/√2) = 0.707 cation = 0.707 anion = 0.707 90 Exercises Find, using symmetry, the orbitals for • the trimethylenemethane H2C=C(CH2)2 • the pentadiene* • the cyclopentadiene* *Explain the origin of the solutions x=±1 for the pentadiene *Explain the origin of the golden number solutions for the cyclopentadiene 91 C2 Trimethylene-methane Symmetry orbitals : F0 1/√3 (f1+ f2+f3) C1 C3 MOs } 1/√2 [F0 + 1/√3 (f1+ f2+f3)] 1/√2 [F0 - 1/√3 (f1+ f2+f3)] 1/√2 (f2-f3) 1/√2 (f2-f3) √(2/3) f1+ 1√6 ( f2+f3) √(2/3) f1+ 1√6 ( f2+f3) Energies 0 C0 energies ± √3 92 C2 Trimethylene-methaneC1 C0 C3 1/√2 [F0 - 1/√3 (f1+ f2+f3)] √(2/3) f1+ 1√6 ( f2+f3) 1/√2 [F0 + 1/√3 (f1+ f2+f3)] 1/√2 (f2-f3) 93 Cyclopentadienyl radical and ions 3 Sym 1 2 3 1 -x 1 0 2 +2 -x 1 3 0 1 -x+1 4 2 5 1 -x2(1-x)+2(1-x)+x=0 x3-x2-x+2=0 x=2 solution (x-2) (x2-x-1) x= (1±√5)/2 The antisymmetric solutions are those from butadiene 94 Pentagon, icosahedra - =-1.618b F=1/ =0.618b 2b 95 Pentagon, icosahedra -1.618b This level is bonding, preferably filled. 0.618b 2b 96 The anion is stable (aromatic) The cation is not (antiaromaticJahn-Teller situation) Thorium+4 97 Exercise: naphtalene 1) Write the Huckel determinant for each symmetry group of the naphatlene. 2) Solve the SS secular determinant 3) Deduce from the SS energy levels those for AS 4) What must be the levels for SA and AA solutions ? 5) Draw the non-bonding level of the pentadienyl radical (C5 chain)? 6) Explain from there what are the levels SS and AS at x=±1 98 naphtalene SS 1 2 3 1 -x+1 1 0 2 +1 -x 2 3 0 1 -x+1 SS -x (1-x) 2-3(1-x)=0 (1-x)(-x2+x+3)=0 x=1 solution (-x2+x+3)=0 x= (1±√13)/2x = 2.30277 and -1.30277 AS solutions have opposite energies (alternant) -1, x=-2.30277 and +1.30277 The SA and AA solutions are the 4 MOs from butadiene ± 0.618 and ± 1.618 The levels at ±1 are duplication of the non bonding MO from the 99 pentadienyle in-phase and out of-phase. Exercises • Calculate the energy for C3 and C5 – Linear or cyclic – Positively charge and negatively charged. • Calculate the MO energies for the biphenyle. Repeat the calculation assuming that the central bond is only half of a C=C bond. 100