溶凝膠法製做玻璃
advertisement
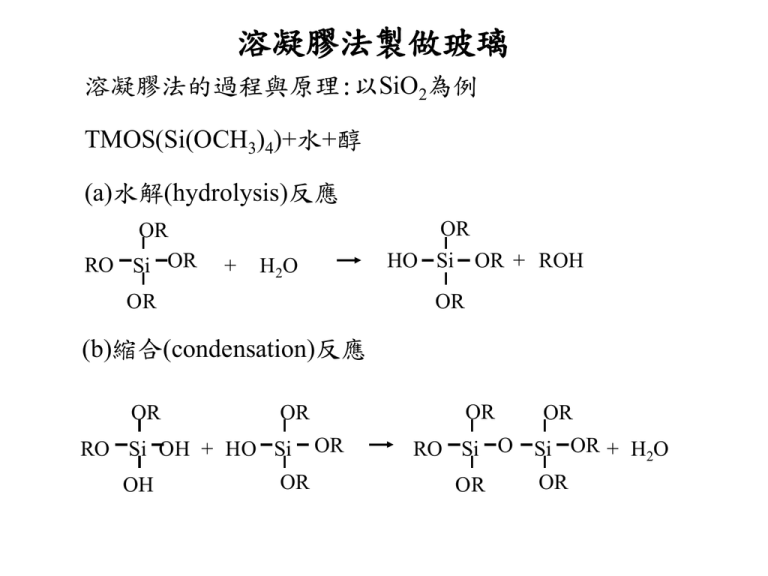
溶凝膠法製做玻璃 溶凝膠法的過程與原理:以SiO2為例 TMOS(Si(OCH3)4)+水+醇 (a)水解(hydrolysis)反應 OR RO Si OR + OR HO Si OR + ROH H2O OR OR (b)縮合(condensation)反應 OR RO Si OH + HO Si OH OR OR OR OR OR RO Si O Si OR + H2O OR OR (c) 多縮合反應(polycondensation) OH HO OH Si O Si OH + 6Si(OH)4 HO HO OH HO Si O O H H OH O HO Si O Si O OH Si OH OH O Si O Si OH + 6(H2O) O HO O O H H HO Si O O Si OH H OH OH 聚合反應 單體 (monomer) 顆粒 (particle) 鏈狀結構 (chain) 三維網狀結構 (3D network) 不同環境下的聚合反應 (1) Far from gel point 10nm 10nm (2) Near from gel point (3) Gel point (acid-catalyzed) (base-catalyzed) 溶凝膠過程與溫度之關係 溶凝膠的不同製程與結果 xerogel film dense film heat Metal alkoxide solution xerogel wet gel evaporation hydrolysis condensation dense ceramics heat aerogel uniform particle sol precipitating furnace ceramic fiber 溶凝膠法的優缺點 優點: (1) 均質性與高純度。 (2) 節省能源,減少蒸發的損失與空 氣的汙染、較純的樣品、避開相 變、結晶的過程。 缺點: (1) 原料昂貴。 (2) 凝膠的收縮量大。 (3) 殘留孔穴、氫氧基、碳。 影響成膠時間的因素 1. 溶液酸鹼值 2. 溫度 3. 矽前驅物與水的莫耳比 4. 矽前驅物的分子量 5. 醇類與水的體積比 6. 其他溶劑與添加物 solvent Methanol (CH3OH) Formamide(甲醯胺,HCNOH2) gelling time (hrs) 8 6 Dimethyl-formamide(二甲基甲醯胺, C3H7CNO) 28 23 Acetonitrile(甲基氰, H3CCN) 41 Dioxane(二氧六環, C H O ) 4 8 2 熟化過程(Aging process) acid-catalyzed base-catalyzed particulate silica gels high solubility particulate silica gels low solubility 緻密化(Densification) Flow Chart of the two methods used to vary the pore characteristics of the gel silica matrices Alumina gel ORMOSILS (Organically Modified Silicates) Si(OR)4+R2Si(OR)2+yR′Si(OC2H5)3 where R is alkyl(烷基) group( -CH3), R′ is alkylene group(烯 烴基 -(CH2)n), y is organofunction group such as -(CH2)3NH2, -(CH2)3NHCOONH2, - (CH2)3S(CH2)2CHO. Basic NMR Interactions in Solids NMR: Nuclear Magnetic Resonance The Hamiltonian of the interaction of the nucleus with external magnetic field B0 and its environment: H=HZ + HQ + HC + HD Where HZ is the Zeeman interaction, HQ is the quadrupole interaction, HC is the chemical shift interaction, and HD is the magnetic dipoledipole interaction. Zeeman interactio n H Z = -μ • B0 E = -γB0 m, where m = -I, I - 1, , - I + 1, - I resonance frequency ν 0 = γB0 / 2 π 原子核種 自旋 自然界含量(%) 磁場7T時 的共振頻率(MHz) 1H 1/2 99.9 300.1 6Li 1 3/2 7.6 92 44.1 116.6 11B 3 3/2 19.9 80.1 32.2 96.3 17O 5/2 0.038 40.7 27Al 5/2 100 78.2 29Si 1/2 4.7 59.6 31P 1/2 100 121.4 51V 7/2 99.7 78.9 69Ga 3/2 3/2 60 40 72.0 91.5 7Li 10B 71Ga FT FT Quadruploe interaction- first order 1 1 1 ν m↔m-1 = ν 0 - ν Q [ (3 cos 2 θ - 1) - η sin 2 θ cos 2φ]( m - ) 2 2 2 3Qcc where the quadrupole coupling constant ν Q = 2I(2I - 1) and η is the asymmetry parameter (0 ≤η ≤1) First order quadrupole powder pattern for spin I=3/2 Quadrupole Interaction- second order R [A (φ) cos 4 θ + B(φ) cos 2 θ + C(φ)] 6ν0 3 2 where R = ν Q [I(I + 1) - ], 4 27 9 3 A (φ) = - - ηcos2φ - η2cos 2 2φ, 8 4 8 30 1 3 B(φ) = - - η2 + 27cos2φ + η2cos 2 2φ, 8 2 4 3 1 1 3 C(φ) = - + η2 + ηcos2φ - η2cos 2 2φ. 8 3 4 8 ν -1/2 ↔1/2 = ν 0 - Second order quadrupole powder pattern for central transition of a spin I=3/2 電腦模擬參數 QCC sQcc η sh Weight(%) BO3 2.55MHz 180KHz 0.15 0 60.4 BO4 0.2MHz 0 0.1 0 39.6 3Si+1B 4Si 27Al MAS spectrum of 9Al2O3-2B2O3 B0=40T 11B MAS spectrum of borosilicate glass B0=14.1T Chemical shift interaction H CS = γ I • σ • B0 where I is the spin - angular momentum, σ is the chemical shift tens or, and B0 is the external magnetic field . Chemical shift powder pattern ν=ν0 [(1 - σ33 )sin 2 θsin2 φ+ (1 - σ22 )cos 2 θsin2 φ+ (1 - σ11 )cos 2 θ] =ν0 [1 - σis o - σax (3cos 2 θ- 1) - σanis o s in 2 θcos2φ] where the angle θ and φ are the polar angles of the field B0 with respect to the principal axes of the chemical shift tens or, and σ11, σ 22 , σ33 are the principal values of the chemical shift tens or which are labeled so that σ33 ≤ σ 22 ≤ σ11, 1 1 1 σiso = ( σ11 + σ 22 + σ33 ), σ ax = (2σ11 - σ33 - σ 22 ), σ aniso = (σ 22 - σ11 ). 3 6 2 ν1 = ν 0 (1 - σ11 ), ν 2 = ν 0 (1 - σ 22 ), ν 3 = ν 0 (1 - σ33 ). Magnetic dipole-dipole interaction 1 μ i • μ j 3(μ i • rij )(μ j • rij ) H D = ∑[ 3 ] 5 2 i ≠j rij rij 2 ( 1 3 cos θij ) 1 2∑ ≈ γi γ j [ (3Iiz I jz - Ii • I j )] 3 4 r i ≠j ij In practice, it is very difficult to carry out a calculation of the lineshape due to dipole-dipole interaction. An excellent approximation for many cases is made by using a normalized Gaussian shape function given by 1 - ( ν - ν0 )2 G ( ν) = exp[ ] 2 Δ 2π 2Δ where 2Δ is equal to the peak to peak width of the derivative of G( ν ). 7 Tesla 14 Tesla 21.1 Tesla The 45 Tesla Hybrid superconducting magnet of 11.5 tesla with a resistive magnet of 33.5 tesla Strength 45 tesla Type Hybrid Bore size 32 mm (~1.25 inches) Online since December 1999 Cost $14.4 million Weight 31,752 kg (35 tons) Height 6.7 meters (22 feet) Operating temperature -271 ° C (-456 ° F) Water used per minute 15,142 liters (4,000 gallons) Power required 33 MW Magic Angle Spinning (MAS) probe rotor Second order quadrupole interaction Chemical shift interaction The structural groups of alkali silicate glasses determined from 29Si MAS-NMR (Journal of Non-Crystalline Solids 127 (1991) 53-64) 29SI 29Si MAS-NMR spectra of sodium silicate glasses. MAS-NMR spectrum of sodium metasilicate glass. Experimentally determined Q, distribution in lithium (△), sodium (□) and potassium (○) silicate glasses as a function of moll% of alkali oxide. Fitted lines were calculated from equilibrium constants shown in table. , Q 4; , Q3 ; , Q 2; , Q1 , , Q0. Conclusion: The detailed distribution of the structural units Qn in binary silicate glasses was determined by means of the MAS-NMR technique. The equilibrium of the following types were found apparently to govern the concentrations of Qn species, 2Qn Qn-1 + Qn+1 (n = 3, 2, 1), of n = 3, 2, 1 for sodium and potassium and n = 3, 2 for lithium silicate glasses in limited composition ranges. The agreements with the thermodynamic data were quantitative in the sodium and potassium silicates but only qualitative in the lithium silicate. The chemical shift for all Qn, species depends linearly on the composition and the slopes are less for Qn, with smaller n. The linear relations between the averaged chemical shift and the theoretical optical basicity strongly suggest the potential use of the 29Si chemical shift as a scale for the basicity of the system. Structure of sodium aluminoborate glasses study by NMR (Solid State Nuclear Magnetic Resonance 27 (2005) 37–49) 27Al B=18.8T 11B B=14.1T 17O B=14.1T Random mixing model: In a random mixing model without any constraints. 4–4 avoidance model: In this model, connections between tetrahedral network units, [4]M–[4]N (avoidance of [4]Al–O–[4]Al, [4]Al–O–[4]B and [4]B–O–[4]B species) are unfavorable, involving only trivalent cations (B and Al). oxygen containing [5,6]Al three-coordinated Conclusions: Details of linkages such as [4]Al–O–[4]Al, [3]O(2[5,6]Al,[4]Al), [4]Al–O–[4]B, [4]Al–O–[3]B, [5,6]Al–O–[3]B, [4]Al–O–[4]B, [4]B–O–[3]B and [3]B–O–[3]B and B-NBO can be distinguished. The fractions of oxygen species can be calculated with the known fraction of B and Al species based on random mixing and mixing considering 4–4 avoidance (avoidance of [4]Al–O–[4]Al, [4]Al–O–[4]B and [4]B–O–[4]B species). All of the glasses in this study show high degrees of bond regularity (higher fractions of Al–B pairs than random) resulting from the ‘‘maximum 4–4 avoidance’’. However, the significant amounts of [4]Al–O–[4]B suggests that the [4]Al–O–[4]B is energetically less unfavorable than [4]Al–O–[4]Al and [4]B–O–[4]B. A better approach to predicting the oxygen speciation for the glasses containing significant amounts of [5,6]Al involves grouping two [5,6]Al species. The result strongly suggests the presence of [3]O(2[5,6]Al, [4]Al).