Heme Plane Shift upon O 2 Binding
advertisement

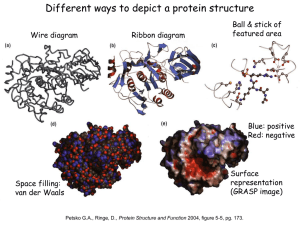
Chapter 7 Hemoglobin: Portrait of a Protein in Action Heme Allows Myoglobin and Hemoglobin to Bind Oxygen • Heme = Fe2+ (ferrous) + Protoporphyrin • Protoporphyrin = 4 pyrrole rings + 4 methine bridges + 4 methyl groups + 2 vinyl groups + 2 propionate side chains • 5th coordination site occupied by the imidazole ring of proximal histidine of myoglobin (or hemoglobin) • 6th coordination site occupied by oxygen Max Perutz &John Kendrew First protein crystal structure in 1950s.1MBD.pdb Heme Plane Shift upon O2 Binding by rearrangement of Fe electron Maintenance of Heme Functionality Ferrous Oxymyoglobin [Fe2+/O2] O Ferrous Deoxymyoglobin [Fe2+] + O2 toxicity Ferric Oxymyoglobin [Fe3+/O2-] X Metmyoglobin [Fe3+] + O2(unable to bind O2) • Oxygen must leave as dioxygen rather than superoxide. Why? • Reversible oxygen binding and storage • The hydrogen bonding between the distal histidine and oxygen stabilizes the ferric (Fe3+) form of oxymyoglobin and does not allow superoxide release. • Oxygen can be released only from the ferrous (Fe2+) form of oxymyoglobin. Tetrameric Structure of Hemoglobin Max Perutz, horse heart 1A3N.pdb • Hemoglobin a Chain vs. Myoglobin : 25% Identity • Hemoglobin b Chain vs. Myoglobin : 24% Identity • Hemoglobin subunits and myoglobins share an evolutionarily conserved structural pattern called “globin fold”. Oxygen Binding Properties of Myoglobin and Hemoglobin Mb : Hyperbolic P50 = 2 torr Hb : Sigmoidal P50 = 26 torr • 2,3-bisphosphoglycerate in red blood cell significantly lowers oxygen binding affinity of hemoglobin. • 4 independent oxygen bindings ? • Cooperative bindings ? Loading & Unloading of Oxygen by Hemoglobin X10 than myoglobin, X1.7 than noncooperative protein • O2 Uptake by Hemoglobin in Lung (pO2 ~100 torr; 98% occupancy) • O2 Release in Typical Peripheral Tissues (pO2 ~20 torr; 32% occupancy; 66% Release) • O2 Unloading in Resting Muscle (pO2 ~40 torr; 77% occupancy; 21% Release) • O2 Unloading in Exercising Muscle (pO2 ~20 torr; 32% occupancy; 67% Release) O2 Binding Changes the Quaternary Structure of Hemoglobin • 15 degree rotation of a1b1 dimer and a2b2 dimer upon oxygen binding • Overall structure of dimer themselves is relatively unchanged. • The interface between a1b1 dimer and a2b2 dimer is significantly changed. • Deoxyhemoglobin : T (for Tense) State; Stronger Inter-Subunit Interactions • Oxyhemoglobin : R (for Relaxed) State; Weaker Inter-Subunit Interactions • In R state, oxygen binding sites are free of strain and show higher affinity Hemoglobin Cooperativity (1) Concerted Model (MWC Model) • Oxygen Binding Affinity : T state < R state • Oxygen binding shifts the quaternary structure of hemoglobin from T to R. • Oxyhemoglobin favors R state, whereas deoxyhemoglobin is in T state. • This model postulates that all 4 subunits are in the same conformation. Hemoglobin Cooperativity (2) Sequential Model • Oxygen Binding Affinity : square state ( ) < quarter circle state ( ) • Oxygen binding modulates the tertiary structure of the neighboring subunits. • This model postulates that the individual subunits can have different conformations. Hemoglobin Cooperativity (3) The Truth ? • Neither the concerted model nor the sequential model is fully accurate. • Oxygen Binding Affinity Hemoglobin-[O2]0 (T) : Hemoglobin-[O2]3 (R) = 1 : 20 (Concerted Model Works !!!) • Oxygen Binding Affinity Hemoglobin-[O2]0 (T) : Hemoglobin-[O2]1 (T) = 1 : 3 (Sequential Model Works !!!) Oxygen Binding to Heme Changes Interface Structures • Oxygen binding causes T to R quaternary structure changes. HOW ??? • Oxygen binding induces heme plane movement. Heme plane movement prompts conformational changes of hemoglobin subunit via proximal histidine residue, which is the 5th coordination site of the heme iron. The C-terminal end of the individual subunit, which is located in the inter-subunit space, is relocated upon oxygen binding. The structural transition at the iron in one subunit is directly transmitted to the other subunits changing the interface between the ab dimers. Cooperative oxygen binding by hemoglobin Specific Binding of 2,3-BPG to T-State Hemoglobin • • • • • • • The T state of hemoglobin is highly unstable. (themodynamically not favored…) R T transition is essential for efficient oxygen unloading. Thus, an additional mechanism is necessary to promote R T transition !!! In red blood cell, [Hb] = [2,3,-BPG] = ~2 mM. 2,3-BPG can bind to the central pocket located in the center of the tetramer. 2,3-BPG binding pocket can be generated only in the T-state. Thus, 2,3-BPG can stabilize the T form population. Cargo Transport Efficiency of Hemoglobin vs. 2,3-BPG • • • • • • [2,3-BPG]LOW R > T and [2,3-BPG]HIGH R < T (Allosteric Regulation) O2 unloading efficacy 2,3-BPG(-) : 2,3-BPG(+) = 8% : 66% Adult Hemoglobin [ab]2 vs. Fetal Hemoglobin [ag]2 In g chain, serine residue has replaced 143 Histidine residue of b chain, which provides a positive charge critical for optimum 2,3-BPG binding. Thus, 2,3-BPG binding is much less efficient for fetal hemoglobin. Oxygen affinity : fetal hemoglobin > maternal hemoglobin. The Bohr Effect (1) : Hydrogen Ion (pH) • Rapidly metabolizing tissues such as contracting muscle generate large amounts of hydrogen ions. • Decrease of pH from 7.4 to 7.2 increases oxygen unloading efficiency by 11%. • Salt bridges involving Lys40 of a2, His146 of b1 and Asp94 of b1 stabilize the T form of hemoglobin. • Protonation on His146 of b1 enhances salt bridge formation with Asp94 of b1. The Bohr Effect (2) : Carbon Dioxide (1) Constant pH! • Actively metabolizing tissues release large amounts of CO2 resulting in pH decrease. • Carbonic Anhydrase : Carbon Dioxide Carbonic Acid (pKa 3.5) Bicarbonate + H+ • 40 torr CO2 increases oxygen unloading efficiency by 11% at pH 7.2. CO2 decrease the affinity of hemoglobin for oxygen beyond the effect due to decrease in pH The Bohr Effect (3) : Carbon Dioxide (2) • CO2 can react with the N-terminal amino group of the hemoglobin subunits and yield carbamate groups, which produce additional negative charges. • The amino termini reside in the interface of ab dimers. • These CO2-induced negative charges in the N-termini of hemoglobin subunits consequently promote salt bridges formation stabilizing the T form. Carbon Dioxide Deportation • Carbamate yielding reactions contribute to process 14% of the total CO2 released from the peripheries. • The majority of the CO2 released from the peripheries is uptaken by the red blood cells and processed to HCO3-, and eventually exhaled in lung as CO2 via reverse conversion reactions (i.e. HCO3- CO2). Sickle Cell Anemia Sickle-Shaped RBC Deoxyhemoglobin S Hemoglobin Fibers • In Sickle-Cell Anemia, Glu6 of b chain is mutated to Val6 (HbS substitution). • The deoxy HbS b chains can establish aberrant hydrophobic interactions involving Val6 of one b chain and Phe85 and Val88 of another b chain (i.e. between different hemoglobin tetramers). Deoxyhemoglobin polymerization Formation of hemoglobin fibers (i.e. insoluble aggregates of hemoglobin) • The HbS substitution does not alter the properties of oxyhemoglobin because the hydrophobic interaction between Val6 and Phe85/Val88 is not allowed in R state.