File
advertisement

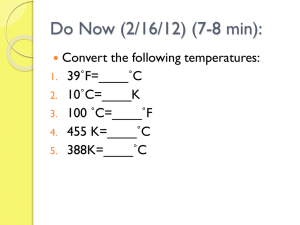
Chapter 14: Heat Chapter Outline • • • • • • • Heat as energy transfer Temperature vs. heat vs. internal energy Internal energy of an Ideal Gas Specific Heat Calorimetry Latent Heat Heat Transfer: conduction, convection, and radiation Heat is a Transfer of energy • Heat always flows from hot to cold. • In the 18th century scientists believed heat was an actual, physical thing that flowed from one object to another. – They pictured it as a fluid and named that fluid caloric • The scientists were never able to detect this fluid. The New Model • Scientists never discovered caloric because heat is not a physical object. • In the 1800s several scientists worked on a new model of heat, one such scientist was an English brewer named James Prescott. • Prescott filled a vessel with water that had paddles in it that would move when a mass was dropped. The Result • What Prescott discovered was that the temperature of the water increased. • Prescott showed that mechanical energy is transferred from one object to another as heat. • What we have been calling the moss-pit is the transfer of heat. Units of Heat • calorie: the energy needed to heat 1g of water 1C • Calorie (aka Kilocalorie aka food calorie): the energy needed to heat 1kg of water 1C • 1 calorie = 4.186J • 1 kilocalorie(kcal) = 4186J • These bottom 2 conversions show how heat IS energy Please Consider the Following • If heat is energy and work is the exchange of energy, then can heat do work? Example 1 • How tall a flight of stairs would a person have to climb to burn off 500 Calories? Assume the person is 60kg. Solution • Step 1 – convert Cal to J • 500kcal (4186J/kcal) = 2.1E6J • So 500 Calories gives us 2.1E6J of work, how high will that work take us? • Step 2 W = mgh • h = W/mg = 2.1E6J / (60kg x 9.8m/s2) = 3600m or over 11,000 ft! Conversation of Energy • Heat factors into the conversation of energy • If any kinetic energy is lost, it is lost as heat. • KEi = KEf + Q, where Q is heat, (why Q? I don’t know) Example • A 3.0g bullet travelling with a speed of 400m/s passes through a tree and slows down to 200m/s. How much heat, Q, is produced and shared by the bullet to the tree? Solution • Step 1: convert to kg • 3.0g = 3.0E-3kg • Step 2: KEi = KEf + Q • • • • Q = KEi - KEf Q = 1/2mvi2 – 1/2mvf2 Q = 1/2m(vi2 – vf2) Q = 180J = 43cal Temperature, Heat, and Internal Energy • The sum total of all the energy of all the molecules in an object is called the internal energy. • Remember, temperature is the average kinetic energy of all molecules in an object. Consider This • If you touch a glass of water that is the same temperature as your hand is there heat transfer? • Does the water have internal energy? Internal Energy of an Ideal Gas • The internal energy of an ideal gas, U, depends only on the temperature of the gas and how many moles of gas there are. • U = 3/2nRT • Again this is for an ideal, monatomic gas. • For a real gas, rotational and vibrational energies would come into play. Heat and Temperature • As heat is put into an object the temperature goes up. • But by how much? • Well that depends… Some questions to ask • Is there a difference to how long it takes to boil a pot of water if it is a little pot or a big pot? • Does it take more or less energy to get heavier molecules moving? • Will it take more heat to get to a higher temperature? The math • Q = mcΔT, where m is mass, T is temperature, and c is called specific heat and is different for every element. • c = Q/mΔT and its units are J/kgCo Heat and conservation of energy • Imagine a completely isolated system where no energy can flow into or out of the system. • In such a system the energy must be conserved. • So, if heat is lost by one part of the system it must be gained by another part. Calorimetry • Heat lost = heat gained • Remember from yesterday, Q = mcΔT • So mc(Tf – Ti) = mc(Tf – Ti) Example • If 200cm3 of tea at 950C is poured into a 150g glass cup initially at 250C, what will be the final temperature T of the mixture when equilibrium is reached, assuming no heat flows to the surroundings? Solution part 1 • We need to find m, c, and ΔT • m: mass is density times volume so m = 200E-6m3 x 1E3kg/m3 = 0.20kg • c = 4186J/kgC (because tea is basically water) • Conservation of energy gives us mteactea(950C – T) = mcupccup(T – 250C) Solution part 2 • mteactea(950C – T) = mcupccup(T – 250C) • (0.20kg)(4186J/kgC)(95 – T) = (0.15kg)(840J/kgC)(T – 25) • Solving for T gives us T = 890C Finding Specific Heats • What could you do to find the specific heat of an unknown substance? What scientists do • They perform what is called calorimetry – They heat the object to a certain temperature. – They quickly place the hot object into an amount of water whose mass and temperature are known. – They record the final temperature of the water to see how much energy was transferred. • Important, when doing this, scientists try to keep the system well insulated from the outside environment. The details • Heat lost by sample = heat gained by water + heat gained by the container • msamplecsampleΔTsample = mwatercwaterΔTwater + mcupccupΔTcup Example • We want to know the specific heat of a new metal alloy that we created. A 0.150kg sample of the new alloy is heated to 5400C. It is then placed in 400g of water at 100C, which is contained in a 200g aluminum calorimeter cup. If the final temperature of the water is 30.50C, what is the specific heat of our alloy? Solution • msamplecsampleΔTsample = mwatercwaterΔTwater + mcupccupΔTcup • (0.150kg)(csample)(540 – 30.5) = (0.400kg)(4186J/kgC)(30.5 – 10) + (0.200kg)(900J/kgC)(30.5 – 10.0) • 76.4csample = (34,300 + 3700)J/kgC • csample = 500J/kgC Bomb calorimeter • A bomb calorimeter is used to measure the heat released when a substance burns or explodes. • It is used to find the calorie content of foods or the energy released by a type of explosive. • A carefully weighted sample of the substance, together with an excess of oxygen at high pressure, is placed in a sealed container (the bomb) which is placed in the water and ignited. Conduction • Heat transfer by molecules colliding • The flow of heat is related to the difference in temperature Q t kA T1 T 2 l • Where l is the distance between the ends of the two objects, A is the area, and k is called the thermal conductivity, which is different for different materials Conductors vs. Insulators • Materials that have a high k transfer heat quickly and are called conductors. • Materials that have a low k transfer heat slowly and are called insulators. Vive la Resistance • Insulators commonly have an R value assigned to them to illustrate how good an insulator they are. • The higher the R, the better the insulator • R = l/k where l is the thickness of the material and k is its thermal conductivity Convection • Heat transfer by the mass movement of molecules from one place to another. • 2 types – Forced: like a furnace blowing hot air into a room – Natural: warm air rises Radiation • Heat transfer that requires no medium at all. • This is how the sun transfers its heat to earth or how IR lamps keep food warm Stefan-Boltzmann equation Q t e AT 4 Where σ is called the Stefan-Boltzmann constant, σ = 5.67E-8W/m2K4 and e is called the emissivity and is between 0 and 1 More on emissivity • Dark objects like black clothing or dark roofs have an emissivity close to 1 and absorb radiation • Light objects like white roofs reflect radiation Going Tanning • The sun sends 1350J of energy per second per square meter at a right angle to the earth. • About 1000W/m2 reaches the surface. • The following equation can be used to find how much radiation an object absorbs from the sun. Q t (1000 W / m ) eA cos 2