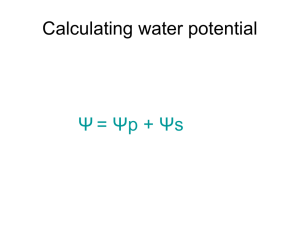Osmosis Review + Virtual Lab AP BIO 2014
advertisement

Diffusion/Osmosis/Water Potential Lab Directions • Read through the background information covered in the next few slides. This is essential knowledge to complete this assignment. • Write the your answers to the questions in your CLASS NOTEBOOK. • Be sure to separate questions by section. For example: – Part 1 Diffusion 1. 2. 3. – Part 2 Osmosis 1. • • • • • Answer A.) blah…. B.) ehhh. C.) huummm.. A.)… B.)…C)… …… Must be neatly organized and written. You do not need to rewrite the questions. YOU DO need to recreate the entire chart for Part 3 and generate a graph. Show your work for all math problems (rewrite equations, ect.) Questions based on the concepts and skills in this assignment/lab will be part of your Chapter 4/5 Test) • Diffusion Molecules are in constant motion and tend to move from regions where they are in higher concentration to regions where they are less concentrated. Diffusion is the net movement of molecules down their concentration gradient. Diffusion can occur in gases, in liquids, or through solids. An example of diffusion in gases occurs when a bottle of perfume is opened at the front of a room. Within minutes people further and further from the source can smell the perfume. • Osmosis is a specialized case of diffusion that involves the passive transport of water. In osmosis water moves through a selectively permeable membrane from a region of its higher concentration to a region of its lower concentration. The membrane selectively allows passage of certain types of molecules while restricting the movement of others. • The solute concentration in the beaker is higher than that in the bag, and thus the water concentration is lower in the beaker than in the bag. This causes water to move from the bag (left) into the beaker (right). • There are often several different types of molecules in a solution. The motion of each type of molecule is random and independent of other molecules in the solution. Each molecule moves down its own concentration gradient, from a region of its high concentration to a region of its low concentration. • Though the net movement of molecules is down their concentration gradient, at any time molecules can move in both directions as long as the membrane is permeable to the molecule. Keep this in mind while you take a closer look at the beaker on the next slide • Notice that the starch molecules are too large to pass through the pores in the membrane. The iodine molecules move across the membrane in both directions, but their net movement is from the bag, where their concentration is higher, into the beaker, where their concentration is lower. The iodine combines with starch to form a purplish-colored compound. • The net movement of water is into the beaker Movement of Molecules Inside a Cell • Like dialysis bags, cell membranes are selectively permeable. As you view the next animation, watch for the selective property of the cell membrane and the two-way diffusion of molecules. Finally, notice the net movement of the molecules. • The movement of water is influenced by the solute concentrations of the solutions. Let's review the different types of solutions. Types of Solutions Based on Solute Concentration: The terms hypotonic, hypertonic, and isotonic are used to compare solutions relative to their solute concentrations • In the illustration, the solution in the bag contains less solute than the solution in the beaker. The solution in the bag is hypotonic (lower solute concentration) to the solution in the beaker. • The solution in the beaker is hypertonic (higher solute concentration) to the one in the bag. Water will move from the hypotonic solution into the hypertonic solution • In this illustration the two solutions are equal in their solute concentrations. We say that they are isotonic to each other. Water Potential • YOU MUST UNDERSTAND the concept of water potential. – Biologists use this term to describe the tendency of water to leave one place in favor of another. Water always moves from an area of higher water potential to an area of lower water potential. Water Potential • Water potential is affected by two factors: pressure and the amount of solute. – For example, imagine a red blood cell dropped into distilled water. Water will move into the red blood cell and cause the cell to expand, stretching the flexible membrane. At some point, the pressure of the incoming water will cause the cell to pop, just like an over-filled balloon. Water Potential • If a plant cell is placed in distilled water, water will enter the cell and the cell contents will expand. However, the elastic cell wall exerts a back pressure, which will limit the net gain of water. Water potential is calculated using the following formula: Water potential () = pressure potential (p) + solute potential (s) Solute potential (s): The effect of solute concentration. Pure water at atmospheric pressure has a solute potential of zero. As solute is added, the value for solute potential becomes more negative. This causes water potential to decrease also. In sum, as solute is added, the water potential of a solution drops, and water will tend to move into the solution Pressure potential (p) In a plant cell, pressure exerted by the rigid cell wall that limits further water uptake. For this ‘lab’ we will be using bars as the unit of measure for water potential; 1 bar = approximately 1 atmosphere. Water Potential • Other units of measurement: megapascals (Mpa) 1 MegaPascal = 10 atm = 145.1 psi • Water potential for pure water: 0 • Anything that lowers the “free energy”of water lowers it potential. -dissolved solutes Water potential = pressure potential + solute potential Clarifying Water Potential Values • (2) Factors to consider: p = pressure potential (outside & inside) s = solute potential system = p + s SO…… p results in “+” value p results in “-” value Water Potential Values • High water potential (+Value): - less solute - more water - (hypotonic) • Zero (0) Value: - Pure water • Low water potential (-Value): - More solute - less water - (hypertonic) ****Water will move across a membrane in the direction of the lower water potential**** Calculating Solute Potential Ψs = – iCRT • Variables involved: i, C, R, T i = ionization constant: NaCl = 2.0 (Na+ & Cl-) **for sucrose it will be 1.0 (it doesn’t ionize) C = Molar concentration T = Temperature: ° K R = pressure constant (R = 0.0831 liter bars/mole K) R will always be the same. T will be given to you, but must be in ° K. T = temperature in Kelvin (273 + °C) Calculating the Solute Potential (s) • s = - iCRT • Sample Calc. A 1.0 M sugar solution @ 22° C under standard atmospheric conditions: s = -(1)(1.0 mol)(0.0831 L · bar )(295K) L s = -24.22 bars mol · K Water Balance-Review • Osmoregulation~ control of water balance – Hypertonic~ higher concentration of solutes – Hypotonic~ lower concentration of solutes – Isotonic~ equal concentrations of solutes • Cells with Walls: – Turgid (very firm) – Flaccid (limp) – Plasmolysis~ plasma membrane pulls away from cell wall Dialysis Tubing Experiment An Artificial Cell Permeable to: monosaccharides & water Impermeable to: Disaccharides The Lab QUESTIONS START HERE For a more detailed, or another explanation, you can visit: http://www.phschool.com/science/biology_place/labbench/lab1/intro.html Part 1 Diffusion • Materials: 500 ml beaker; Dialysis bags; Glucose indicating strips; Glucose/starch solution; dilute iodine solution (actual lab uses IKI, potassium iodide solution) • In this activity, you fill a dialysis bag with a sugar/starch solution and immerse the bag in a dilute iodine solution. Water, sugar, starch, and iodine molecules will all be in motion, and each molecule will move to a region of its lower concentration, unless the molecule is too large to pass through the membrane. Your task is to determine relative size of the various molecules and gather evidence of molecular movement. – Hint: One piece of information that will help you is to recall that when iodine comes in contact with starch, it changes from an orange-brown color to blueblack. 1. 2. 3. Create an illustration of this lab set-up. Based on what you have learned, generate a hypothesis for the movement of (a) glucose, (b) starch, and (c) iodine across the membrane. What physical evidence (the data you need to collect) will you use to determine the movement of (a) glucose, (b) starch, and (c) iodine across the membrane. Part 2 Osmosis • Materials: 6x500 ml beaker; 6 Dialysis bags; Scale; Distilled water; Sucrose (molar mass = 342 g/mol); • In this activity you investigate the relationship between solute concentration and water movement by filling six different dialysis bags with increasing concentrations of sucrose (pure water; 0.2 M; 0.4 M; 0.6 M; 0.8 M; & 1.0 M) and placing the bags into distilled water. • After the time for the experiment has elapsed, will need to determine and quantify the movement of water for each of the beaker/dialysis tube set-ups in order to explain the relationship between solute concentration and water movement. 1. Illustrate/draw the set-up for this experiment. 2. Generate a hypothesis for this experiment. 3. How will you quantify/measure net water movement for each beaker/dialysis tube set-ups? 4. Knowing that the molar mass of sucrose is 242 g/mol, how would one go about making a liter of 1 M sucrose solution? Part 3 • This activity is very similar to Part 2, except that you use cores from potatoes instead of dialysis bags. You submerge the cores in solutions of varying sucrose concentrations. 1. On the next slide is a chart filled in with data for you. You need to recreate this chart. Using the data given, calculate the change in mass, & % change (Massf-Massi/Massi x 100) for each potato/solution set-up. 2. Graph the % Change in Mass (X axis) against the sucrose molarity (Y axis). – B. Identify the independent and the dependent variable. 3. Using your graph, what is the molar concentration of the potato core? Explain how you know. – Since you know that the pressure potential of the surrounding solution in an open beaker is zero, you can now calculate the water potential. (use this in part 4) Solution Temperature Potato Cylinders Solution Initial Temp Final Temp Initial Mass Final Mass Change in Mass (g) Water 21 21 1.5 2.1 0.2 M 21 21 1.5 1.6 0.4 M 21 21 1.5 1.3 0.6 M 21 21 1.5 1.2 0.8 M 21 21 1.6 1.1 1.0 M 21 21 1.5 0.9 % Change in Mass Part 4: Calculation of Water Potential from Experimental Data • (Since you know that the pressure potential of the surrounding solution in an open beaker is zero, you can now calculate the water potential. ) 1. In this exercise, use the value for the molar concentration of the potato cores that you obtain in Exercise 3 to determine the water potential for the potato cells. – Ψs = – iCRT – Ψ = Ψp + Ψs Post-Questions 1. What is the symbol for water potential? 2. What is the symbol for pressure potential? 3. What is the symbol for solute potential (aka osmotic potential)? 4. What is the equation for calculating water potential? 5. What is the equation to find solute potential? Post-Questions 6. Suppose you have an artificial cell that was permeable to monosaccharides and impermeable to disaccharides. What would happen to the cell if it had 0.80 M maltose and 0.85 M fructose in it and was placed in a solution containing 0.45 M glucose, 0.65 M fructose, and 0.40 M sucrose. a.) Which direction would the water flow? b.) Which area has a higher water potential? c.) What would happen to the concentration of the maltose inside the cell (increase, decrease, remain the same)? 7. What is the ionization constant (i) for sucrose? Post-Questions 8. Why don't red blood cells pop in the bloodstream? 9. The molar concentration of a sugar solution in an open beaker has been determined to be 0.3M. Calculate the solute potential at 27 degrees. Round your answer to the nearest hundredth. 10.The pressure potential of a solution open to the air is zero. Using the solute potential of the solution from the question above, calculate the water potential. Post-Questions 11. If inside of a cell has a water potential of -24 bars and outside the cell has a water potential of -5 bars, how will water move? 12. If a cell’s ΨP = 3 bars and its ΨS = -4.5 bars, what is the resulting Ψ? 13. The cell from question above is placed in a beaker of sugar water with ΨS = -4.0 bars. In which direction will the net flow of water be? 14. The original cell from question # 12 is placed in a beaker of sugar water with ΨS = -0.15 MPa (megapascals). We know that 1 MPa = 10 bars. In which direction will the net flow of water be? Post-Questions 15.The value for Ψ in root tissue was found to be -3.3 bars. If you take the root tissue and place it in a 0.1 M solution of sucrose at 20°C in an open beaker, what is the Ψ of the solution, and in which direction would the net flow of water be? 16.NaCl dissociates into 2 particles in water: Na+ and Cl-. If the solution in question 4 contained 0.1M NaCl instead of 0.1M sucrose, what is the Ψ of the solution, and in which direction would the net flow of water be?