The Behavior of Gases, Chapter 14

The Behavior of Gases,
Chapter 14
(Unit 1)
Mr. Samaniego
Lawndale High School
California Standards
Gases and Their Properties
4. The kinetic molecular theory describes the motion of atoms and molecules and explains the properties of gases. As a basis for understanding this concept: a. Students know the random motion of molecules and their collisions with a surface create the observable pressure on that surface.
c. Students know how to apply the gas laws to relations between the pressure, temperature, and volume of any amount of an ideal gas or any mixture of ideal gases.
Thinking Map
Draw a Circle Map on Gases.
Gases
Phases of Matter
Direct and Inverse Relationships
As more people watch football, less watch tv shows.
Inverse
More people listen to an artist as they become more popular.
Direct
The more money you make, the more money you spend.
Direct
Inverse vs. Direct
Which relationship is direct or inverse?
Pressure vs.
Temperature
3,0
2,0
1,0
0,0
0
Direct
200 400 600
Temperature (K)
800
300
250
200
150
100
50
0
0,0
1000
3,0
Volume vs. Temperature
2,0
Direct
1,0
0,0
0 200 400 600
Temperature (K)
800 1000
Pressure vs. Volume
0,5
Inverse
1,0 1,5
Volume (L)
2,0 2,5 3,0
The Gas Laws
Boyle’s Law
P
1
V
1
P
2
V
2
GayLussac’s Law
V
1
T
1
V
2
T
2
P
1
T
1
P
2
T
2
Combined Gas Law
Ideal Gas Law
P
1
V
1
T
1
P
2
V
2
T
2
PV nRT
Temperature
There are three common units temperature is measured in:
___________ ( o C), Fahrenheit
( o F), and _________ (K)
Temperature
The important temperature unit in describing Gas behavior is
___________ (K).
Conversion:
Kelvin = Celsius + _____
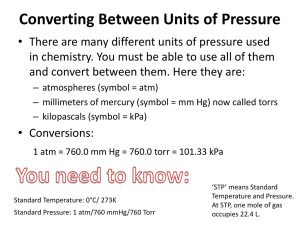
Pressure
There are five common units pressure is measured in:
____________ (kPa),
______________ (atm), mm of mercury (mm Hg), Torr (Torr), and pounds/inch 2 (lbs/in 2 )
Conversion Factors
101.325
___ mm Hg = 760 Torr = 14.7
lbs/in 2
Amount
There are some common unit amounts is measured in :
________ (mol), particles, atoms, etc.
The variable n is used for # of moles
The conversion is:
1 mol = ____________ (anything)
Volume
There are three common units volume is measure in:
_________ (L), milliliters
(mL),
______ _____________ (cm 3 )
Conversion Factors
1 L= ______ mL= 1,000 cm 3
Gas Constant, R
There are different ways of expressing the gas _________ R in solving gas problems. It only depends on the
_______ being used. As long as the units match , the right value is being used.
R = 8.31 L*kPa/(mol*K) = 0.0821 L*atm/(mol*K)
LOGIC with Volume/Pressure
• If volume __________, then the pressure will __________.
LOGIC with Pressure
STP: Standard Temperature and Pressure,
T = 0 o C
P = 1 atm = 101.325 kPa = 760 Torr
Strategies in Problem Solving:
Gas Problems
Strategies in Problem Solving:
Gas Problems
Identify Conversions Calculator known/ uknown values must include proper units!!!
use
LOGIC
(You can do it!) must have unit agreement multiply across top divide across bottom
Poster Design
• Choose 1 Gas Law
• Name it and show the formula
• Include 1 drawing
• Describe what happens: “If temperature increases…”
• 5 points
Warm-up
(2-8-12/2-9-12)
Copy problems and answers into notebook:
1.
What do the following items measure?
a) P b) V pressure volume c) n
Amount (in moles) d) R
Gas constant e) T temperature
2.
Which temperature unit must be used in Gas Law calculations?
Kelvin
3.
How do you convert degrees Celsius to Kelvin?
Add 273 (to Celsius)
Benchmark Reflection
Warm-up
(2-9-12/2-10-12)
Copy problems and answers into notebook:
1.
What happens to the volume if the pressure increases?
It decreases
2.
What happens to the pressure if the temperature decreases?
It decreases
3.
What happens to the temperature if the volume decreases?
It decreases
4.
Describe #’s 1-3 as “Direct” or “Inverse” relationships.
1. inverse 2.
direct 3. direct
5. List the units that apply to each of the following: a) Pressure atmospheres, kPa b) volume liters c) temperature
Kelvin
Warm-up
(2-13-12/2-14-12)
Copy problems and answers into notebook:
1.
What is the standard temperature (see p. 300)?
I. 0 K
II. 0 o C
III. 273 K a) I only b) II only c) III only d) II and III
2. Which Centigrade (Celsius) temperature is the same as 298 K?
a) 25 0 C b) 212 0 C c) 521 0 C d) 596 0 C e) 1058 0 C
3. The pressure exerted by a gas is due to a) the chemical nature of the container; b) the color of the gas; c) the height of the container above sea level; d) the diameter of the gas molecules; e) the collisions of the gas molecules with the walls of the container.
Pressure
• What is pressure created by?
The random collision of molecules and their collisions with a surface.
(Standard 4a)
Boyle’s Law
1. A gas has a volume of 12.5 L at 450. kPa pressure. What pressure is needed to change the volume of the gas to 6.25 L?
Boyle’s Law
2. A gas has a volume of 284 mL while at
101.325 kPa. What new volume will it have if the new pressure is 25.3 kPa?
Boyle’s Law
3. An unknown pressure is maintained while a gas has a volume of 468 mL. If it expands to 702 mL while the gas has a new pressure of 1.80 atm, what was the original pressure?
Boyle’s Law
4. A gas at 203 kPa pressure and unknown volume changes to 24.5 L while under the new pressure of 305 kPa. What was the original volume?
Boyle’s Law
4b. A gas at 502 kPa pressure and unknown volume changes to 355 L while under the new pressure of 1,500 kPa.
What was the original volume?
Charles's Law
5. A gas has a volume of 91 mL at a temperature of 91 0 C. This gas is kept at constant pressure but its temperature is reduced to 0 0 C. What should the new volume be?
(remember to use Kelvin)
Charles's Law
6. Calculate the original temperature that 350. mL of oxygen has, if it occupies 525 mL, while the temperature is 301 K.
Charles's Law
7. Gas in a tire takes up 12.5 L of space at 22.0
o C.
What new temperature in Kelvin should it have if the new volume is 37.5 L?
GayLussac’s Law
8. The air in a car has a pressure of 1.00 atm while the car is at 20.0
o C. If the air pressure does not escape from the car, what new pressure would it have if it heated to 352 o C?
GayLussac’s Law
9. The air in a sealed container has a pressure of
1.00 atm while at 32.0
o C. What new temperature would it have if the pressure went up to 3.00 atm?
Combined Gas Law
10. An air bubble occupies a space of 1.52 mL while at a depth in the ocean where there is 2.50 atm of pressure and a temperature of 15.3 o C.
Find the volume of the bubble just before it reaches the surface, with a pressure of 1.00 atm and a temperature of 25.0 o C.
Boyle’s Law
11. A scuba diver at 20.0 m experiences a pressure of 2.50 atm. The diver inflates her lungs to 1.8 L and holds her breath as she heads to the surface her lungs are at a constant temperature. What would the volume of her lungs expand to as she breaks the surface where the pressure is 1.00 atm?
Combined Gas Law
12. A balloon is inflated to 1.00 L at 1.00 atm, 27 °C. The balloon rises to an altitude of 10,000 meters where the temperature is
1590 K and the pressure is 0.500 atm.
What is the new volume of the balloon?
Combined Gas Law
13. A balloon is inflated to 3.25 L at 1.00 atm, with an unknown temperature. The balloon rises to an altitude of 10,000 meters where the temperature is 1,380 K and the pressure is 0.409 atm and a volume of 13.5 L. What is the original temperature of the balloon?
Clearing Fractions
Dalton’s Law
Dalton’s Law of Partial Pressures: at constant volume and temperature, the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures of the component gases.
P total
= P
1
+ P
2
+ P
3
+ …
Practice
Determine the total pressure exerted by the following gases: 23.4 kPa of oxygen, 14.8 kPa of nitrogen, and 28.3 kPa of carbon dioxide.
Follow-up Assignment
• Chapter 14, Behavior of Gases p. 439: 3954, and 59 [Don’t write questions, just answers.]