pptx
advertisement

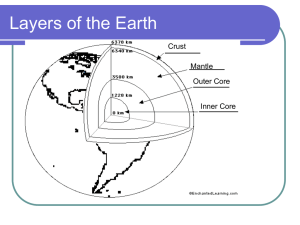
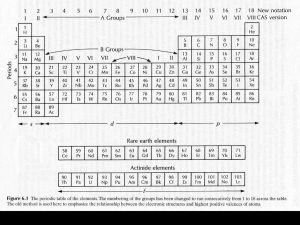
The Embarrassing Details about Geochemical Mass Balance Models for Calculating Mantle Composition (they are trivially simple, but they do give some interesting results if you ask the right questions) The Physical Principal – Conservation of Mass Melt or Crust Bulk Silicate Earth (the hypothetical homogeneous composition of the solid silicate Earth after core formation, but before any other differentiation process) Residue or Depleted Mantle [a] (mg/g) MassBSE Mass of “a” = MassBSE x [a]BSE Mass of element “a” = MassDM x [a]DM + MassC x [a]C + Mass(BSE-DM-C) x [a]BSE The Physical Principal – Conservation of Mass Reservoir A (atmosphere) Reservoir CC (Cont. Crust) Bulk Earth (the hypothetical average homogeneous composition of the Earth) Reservoir OC (Oceanic Crust) [a] (mg/g) MassBE Mass of “a” = MassBE x [a]BE Reservoir BSE Reservoir DM (Depleted Mantle) Reservoir Core Reservoir Moon Mass of “a” = MassA x [a]A + MassCC x [a]CC + MassOC x [a]OC + MassDM x [a]DM + MassCore x [a]Core + MassBSE x [a]BSE + MassMoon x [a]Moon + etc., etc. etc. The Necessary Inputs • Mass and composition of (n-1) reservoirs • If interested only in composition, then all that matters is the mass balance, not the rates of exchange (unless you are interested in how a given reservoir changed in composition with time) • If interested in the radiogenic isotope compositions (e.g. 87Sr/86Sr), then how and when the various reservoirs formed is critical Some Examples of The Questions You Can Ask • • • • • • If the BSE differentiates into continental crust and depleted mantle, given the mass and composition of the continental crust, what portion of the mantle can be as depleted as the source of MORB? How does this change with different estimates of the BSE composition? Do you get the same answer when you use a highly incompatible element (e.g. Ba) and a moderately incompatible element (e.g. Sm)? If the continental crust has the average composition given in the program, could it be 5 times its current size at the same composition? If not, why not? How much Nb must be in the core to explain the non-chondritic Nb/U ratio of the mantle? How does the composition of the EER vary as a function of its size? Can the Moon be the EER? What would its composition be? The mass of the Moon is approximately twice the mass of the continental crust. The mass of the atmosphere is 5 x 1021g of which 0.93% is argon, and most of that argon is 40Ar produced by the decay of 40K over Earth history. 40K decays to 40Ar and 40Ca with a decay constant of 5.54 x 10-10 yr-1, with 11 % of the decay going to 40Ar. The BSE has 240 ppm of K, only 0.012% currently of which is 40K. If all of the 40Ar in the atmosphere came from 40K decay, how much 40Ar remains in the mantle? If all the radiogenic 40Ar is in the atmosphere, what is the K content of the BSE?