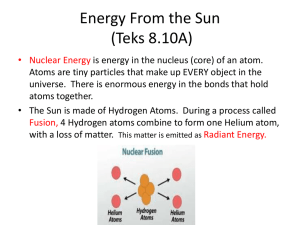
powerpoint - University of Illinois at Urbana

Lecture 1
Discretization of energies
(c) So Hirata, Department of Chemistry, University of Illinois at Urbana-Champaign. This material has been developed and made available online by work supported jointly by University of Illinois, the
National Science Foundation under Grant CHE-1118616 (CAREER), and the Camille & Henry Dreyfus
Foundation, Inc. through the Camille Dreyfus Teacher-Scholar program. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the sponsoring agencies.
Discretization of Energies
Energies are discrete (quantized) and not continuous.
This quantization principle cannot be derived
– rather it should be accepted as physical reality.
We will survey historical developments in physics that led to the important discovery that energies are quantized. The details of each of the experiments or theories are not so important – the conclusion is important.
Quantization of Energy
Classical mechanics: Any value of energy is allowed. Energy is continuous.
Quantum mechanics: Not all values of energy are allowed. Energy is discrete
(quantized).
Black-body radiation
A heated metal emits light.
As the temperature becomes higher, the color of the emitted light shifts from red to white to blue.
How can physics explain this effect?
Light
Wavelength ( λ ) and frequency ( ν ) of light are inversely proportional: c = νλ ( c is the speed of light).
Black-body radiation
What is “temperature”? – translation, rotation, vibrations, etc. of atoms in molecules and solids.
Light of frequency v can also be viewed as an oscillator having temperature.
Equipartition principle: Heat flows from high to low temperature area; each oscillator has the same thermal energy kT at equilibrium.
Black-body radiation
Experimentally, increasing the temperature increases the intensity and decreases the maximum of wavelengths of light.
Black-body radiation
Classical explanation –
Rayleigh-Jeans law – has the limitation.
energy distribution
~ kT / λ 4 ~ kTv 2
Ultraviolet catastrophe.
Rayleigh-Jeans ~ kTv 2 c = vλ
Experimental short wavelength large frequency v
Black-body radiation
Planck could explain the experimental energy density by postulating that the energy of each electromagnetic oscillator is limited to discrete values (quantized) .
E = nh ν ( n = 0,1,2,…).
h is Planck ’ s constant.
Black-body radiation
0 v 2
ν ∞
Each electromagnetic oscillator of a frequency v is given an equal share of energy kT v
Black-body radiation
h ν
0 h ν hν hν hν hν hν hν hν hν h ν h ν h ν h ν h ν
ν ∞ v 2 v 2 × hv /( e hv / kT −1) hv /( e hv / kT −1) Bose-Einstein statistics
Electromagnetic oscillators with smaller frequencies are unaffected, leading to
Rayleigh-Jeans results v
Continuous vs. quantized
Each oscillator has a price tag of hv and
kT may not be enough to buy one hv if v is high kT kT kT kT
Higher frequencies
Planck ’ s constant h
E = nh ν ( n = 0,1,2,…) h = 6.63 x 10
–34
J s. (Joule is the units of energy and is equal to Nm or Newton x meter). The frequency has the units s
–1
.
Because h is small, classical limit works well in so many cases.
In the limit h → 0, E becomes continuous and any arbitrary value of E is allowed. This is the classical limit.
Heat capacities
Heat capacity is the amount of energy one needs to heat up a substance by 1 K. The greater the heat capacity, the more thermally responsive the substance is.
“Heat” is a macroscopic concept of a flow of energies of a collection of oscillators.
Dulong-Petit ’ s law of heat capacity: molar heat capacity of monatomic solids is the same.
Heat capacities
There are N
A
(Avogadro ’ s) number of atoms in a mole of a monatomic solid. Each can act as a three-way oscillator (that oscillates in x , y , and z directions independently).
According to the equipartition principle , each of the three degrees of freedom of an oscillator is entitled to kT energy.
E = 3 N
A kT → C = dE / dT = 3 N
A k = 3 R
R is the gas constant.
Heat capacities
Dulong-Petit ’ s law holds for large T .
For small T , it does not.
The deviation at low T has been explained by
Einstein
Heat capacities
For smaller T , the thermal energy kT ceases to be able to afford the smallest allowed quantum h ν ( ν is the frequency of oscillation).
kT hv hv kT hv kT hv hv hv hv hv hv hv hv hv
…
Small T Large T
Heat capacities
Einstein assumed that there was only one available frequency of oscillation v .
When Debye used a more realistic distribution of frequencies, he obtained a better agreement between theory and experiment.
Atomic & molecular spectra
Colors of matter originate from the light emitted or absorbed by constituent atoms and molecules.
The frequencies of light emitted or absorbed are discrete.
Atomic & molecular spectra
This has been explained by atoms and molecules having discrete energies
( E
1
, E
2
, …) .
When light is emitted or absorbed, an atom or molecule shifts in energy, so hv = E n
– E m
.
Summary
Energies of stable atoms, molecules, electromagnetic radiation, and vibrations of atoms, etc. are discrete ( quantized ). They are not continuous.
Macroscopically observed phenomena, such as red color of hot metals, heat capacity of solids at a low temperature, and colors of matter are all due to quantum effects .
Quantized nature of energy cannot be derived. We should simply accept this.