Chapter_2_notes_rev
advertisement
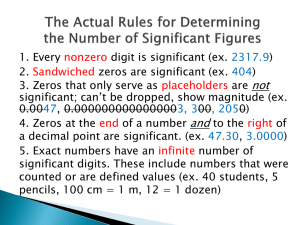
Must have a number and a UNIT SI measurements Base units are defined units based on an object or event in the physical world. Base units are independent of other units. A unit that is defined by a combination of base units is called a derived unit. Volume › space occupied by an object › Cubic meter (m3) Density › Ratio that compares the mass of an object to its volume › Density = mass / volume › Grams/cubic centimeter (g/cm3) Speed › distance / time (meters/second) Temperature is a measure of how hot or cold a substance is relative to other objects Kelvin scale (no degrees used) › Water boils at 373 K › Water freezes at 273 K Celsius scale › Water boils at 100oC › Water freezes at 0oC Converting › oCelsius to Kelvin – oC + 273 › Kelvin to oCelsius –Kelvin - 273 › oCelsius to oFahrenheit – 5/9 (oF -32) › oFahrenheit to oCelsius – 9/5 (oC +32) The Problem 1. Read the problem 2. Be sure that you understand what it is asking you. Analyze the Problem 1. Read the problem 2. Identify what you are given and list the known data 3. Identify and list the unknown 4. Gather information you need from graphs, tables, or figures 5. Plan the steps you will follow to find the answer Solve for the unknown 1. Determine whether you need a sketch to solve the problem. 2. If the solution is mathematical, write the equation and isolate the unknown factor 3. Substitute the known quantities into the equation 4. Solve the equation 5. Continue the solution process until you solve the problem Evaluate the Answer 1. Re-read the problem. Is the answer reasonable? 2. Check your math. Are the units and the significant figures correct? Expresses numbers as a multiple of two factors First factor must follow this rule 1 ≤ 1st factor < 10 When numbers larger than 1 are expressed in scientific notation, the power of ten is positive When numbers smaller than 1 are expressed in scientific notation, the power of ten is negative Express the following in scientific notation a. 700 m e. 0.0054 kg b. 38,000 m f. 0.00000687 kg c. 4,500,000 m g. 0.000000076 kg d. 685,000,000,000 m h. 0.0000000008 kg Express the following quantities in scientific notation i. 360,000 s j. 0.000054 s k. 5060 s l. 89,000,000,000 s Exponents must be the same If they are not the same change the quantities so that the exponents are the same › Move decimal to the left – increase the exponent value › Move decimal to the right – decrease the exponent value › Left › Add › Right › Subtract Add or subtract the number values Exponents will be the same as the original values Multiplication › Exponents do not have to be the same › Multiply the first factors › Then add the exponents Division › Exponents do not have to be the same › Divide the first factors › Then subtract the exponent of the divisor from the exponent of the dividend Take care when determining the sign of the exponent. Conversion Factor › A ratio of equivalent values used to express the same quantity in different units › A conversion factor is always equal to 1 › Change the units without changing the value Dimensional Analysis › Method of problem solving that focuses on the units used to describe matter › Converting from large unit to a small unit the number of units must increase a. Convert 360 s to ms e. Convert 245 ms to s b. Convert 4800 g to kg f. Convert 5 m to cm c. Convert 5600 dm to m g. Convert 6800 cm to m d. Convert 72 g to mg h. Convert 25 kg to Mg How many seconds are there in 24 hours? The density of gold is 19.3 g/mL. What is gold’s density in decigrams per liter? A car is traveling 90.0 km/hr. What is its speed in miles per minute? 1 km = 0.62 miles 24 hr 60 min 60 sec 1 hr 1 min 19.3 g 10 dg 1000 mL mL 1g 1L 90.0 km hr 0.62 miles 1 hr 1 km 60 min = = 86,400 sec 193000 dg L = 0.930 miles min Accuracy refers to how close a measured value is to an accepted value Precision refers to how close a series of measurements are to one another To evaluate the accuracy of experimental data (recorded during experimentation) you can calculate the difference between an experimental value and an accepted value The difference is called an error Percent error is the ratio of an error to an accepted value. Percent Error = error x 100 accepted value Doesn’t matter whether the experimental value is larger or smaller than the accepted value just how far off it was Ignore the plus or minus sign Tolerances – narrow range of error that is acceptable Calculate the percent errors for Student B’s trials. (The accepted value is 1.59 g/cm3). Calculate the percent errors for Student C’s trials. (The accepted value is 1.59 g/cm3). Scientists indicate the precision of measurements by the number of digits they report. The digits that are reported are called significant figures Include all known digits and one estimated digit. Non-zero numbers are always significant Zeros between non-zero numbers are always significant All final zeros to the right of the decimal place are significant Zeros that act as placeholders are not significant. Convert quantities to scientific notation to remove the placeholder zeros Counting numbers and defined constants have an infinite number of significant figures. Determine the number of significant figures in each measurement 508.0 L – 820400.0 L – 1.0200 x 105 kg – 807000 kg – 0.049450 s – 0.000482 mL – 3.1587 x 10-8 g – 0.0084 mL – Calculators often give more significant figures than are appropriate for a given calculation Your answer should have no more significant figures than the data with the fewest significant figures If the digit to the immediate right of the last significant figure is less than five, do not change the last significant figure If the digit to the immediate right of the last significant figure is greater than five, round up the last significant figure If the digit to the immediate right of the last significant figure is equal to five and is followed by a nonzero digit, round up the last significant figure If the digit to the immediate right of the last significant figure is equal to five and is not followed by a nonzero digit, look at the last significant figure. If the last significant figure is an odd digit, round it up. If the last significant figure is an even digit do not round up When adding or subtracting, your answer must have the same number of digits to the right of the decimal point as the value with the fewest digits to the right of the decimal point Round the answer to the same number of places as the fewest in the equation Maintains the same precision as the least precise measurement When multiplying or dividing your answer must have the same number of significant figures as the measurement with the fewest significant figures Creating a graph can help scientists reveal patterns among the data gathered through experimentation We will deal with circle, bar and line graphs Also called pie chart Shows parts of fixed whole Parts are usually percentages Bar graphs show how a quantity varies with factors such as time, location, or temperature The independent variable is plotted on the horizontal (x-axis) The quantity being measured is plotted on the vertical (y-axis) – dependent variable Can also be used to compare data Independent variable is plotted on the x-axis › Variable scientist deliberately changes in the experiment Dependent variable is plotted on the y-axis When points are scattered on the graph a line of best fit must be drawn where an equal number of data points fall above and below the line of best fit If the line of best fit is straight the variables are directly related › The relationship can be described by the slope of the line Line rises to the right = positive slope › Dependent variable increases as the independent variable increases Line sinks to the right = negative slope › The dependent variable decreases as the independent variable increases Slope is Constant Slope = y2 – y1 = ∆y x2 – x1 ∆x Interpolate › Reading data from a graph that falls between measured points Extrapolate › Extend the line beyond the plotted points and estimate values for the variables