Chapter 31:Radioactivity
advertisement

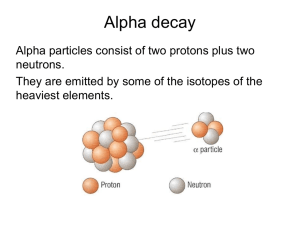
is defined as the spontaneous disintegration of certain atomic nuclei accompanied by the emission of alpha particles, beta particles or gamma radiation. CHAPTER 28: Radioactivity (2 Hours) 1 Learning Outcome: 28.1 Radioactive decay (1 1/2 hours) At the end of this chapter, students should be able to: Explain α, β+, βˉ and γ decays. State decay law and use dN N dt Define activity, A and decay constant, . Derive and use N N 0 e t OR A A0 e t Define half-life and use T1/ 2 ln 2 2 28.1 Radioactive decay Radioactivity is a phenomenon in which an unstable nuclei undergoes spontaneous decay as a result of which a new nucleus is formed and energy in the form of radiation is released The radioactive decay is a spontaneous reaction that is unplanned, cannot be predicted and independent of physical conditions (such as pressure, temperature) and chemical changes. This reaction is random reaction because the probability of a nucleus decaying at a given instant is the same for all the nuclei in the sample. Radioactive radiations are emitted when an unstable nucleus decays. The radiations are alpha particles, beta particles and gamma-rays. 3 28.1.1 Alpha particle () An alpha particle consists of two protons and two neutrons. It is identical to a helium nucleus and its symbol is 4 4 2 He OR 2 It is positively charged particle and its value is +2e with mass of 4.002603 u. When a nucleus undergoes alpha decay it loses four nucleons, two of which are protons, thus the reaction can be represented by general equation below: α A 4 4 A X 2 He Z Z 2 (Parent) (Daughter) ( particle) Y Q • Alpha particles can penetrate a sheet of paper. 4 α particle parent daughter Examples of decay : 218 214 4 Po Pb 84 82 2 He Q 230 226 4 90Th 88 Ra 2 He Q 226 222 4 Ra Rn 88 86 2 He Q 238 234 4 U Th 92 90 2 He Q 5 28.1.2 Beta particle (β) • Two types : a) Beta minus , β + b) Beta plus , β • • A beta particle has the same mass and charge as an electron. Beta particles can penetrate a few mm of Al and their velocity is high (v ~ c). 6 Beta minus (β )-negatively charge. • • Also called as negatron or electron. Symbol; - β or • 0 1 or 0 1 e It is produced when one of the neutrons in the parent nucleus decays into a proton, an electron and an antineutrino. massless, neutral 7 • In beta-minus decay, an electron is emitted, thus the mass number does not charge but the charge of the parent nucleus increases by one as shown below : A Z X Y (Parent) • Examples of A Z 1 (Daughter) 0 1 e Q ( particle) minus decay : 0 Th234 Pa 91 1 e Q 0 Pa234 U 92 1 e Q 0 Bi214 Po 84 1 e Q 234 90 234 91 214 83 8 + Beta plus (β )- positively charge. • • Also called as positron or antielectron. Symbol; + β • or or e 0 0 1 1 It is produced when one of the protons in the parent nucleus decays into a neutron, a positron and a neutrino. massless,neutral 9 • In beta-plus decay, a positron is emitted, this time the charge of the parent nucleus decreases by one as shown below : A Z X (Parent) Y (Daughter) • Example of 12 7 A Z 1 0 1 e Q (Positron) plus decay : N 126C10e v Q 10 28.1.3 Gamma ray () Gamma rays are high energy photons (electromagnetic radiation). Emission of gamma ray does not change the parent nucleus into a different nuclide, since neither the charge nor the nucleon number is changed. A gamma ray photon is emitted when a nucleus in an excited state makes a transition to a ground state. Examples of decay are : 218 214 4 Po Pb 84 82 2 He γ 234 234 0 Pa U 91 92 1e γ 208 208 Ti 81 81Ti γ Gamma ray It is uncharged (neutral) ray and zero mass. The differ between gamma-rays and x-rays of the same wavelength only in the manner in which they are produced; gamma-rays are a result of nuclear processes, whereas x11 rays originate outside the nucleus. 28.1.4 Comparison of the properties between alpha particle, beta particle and gamma ray. Table 28.1 shows the comparison between the radioactive radiations. Alpha Beta Gamma 1e OR +1e 0 (uncharged) Charge +2e Deflection by electric and magnetic fields Yes Yes No Strong Moderate Weak Penetration power Weak Moderate Strong Ability to affect a photographic plate Yes Yes Yes Yes Yes Yes Ionization power Ability to produce Table 28.1 fluorescence 12 28.1.5 Decay constant () Law of radioactive decay states: dN is directly For a radioactive source, the decay rate dt proportional to the number of radioactive nuclei N remaining in the source. i.e. dN Negative sign means the number of N dt dN N dt remaining nuclei decreases with time (28.1) Decay constant Rearranging the eq. (28.1): dN dt N decay rate number of remaining radioactiv e nuclei Hence the decay constant is defined as the probability that a radioactive nucleus will decay in one second. Its unit is s1. 13 The decay constant is a characteristic of the radioactive nuclei. Rearrange the eq. (28.1), we get dN (28.2) dt N At time t=0, N=N0 (initial number of radioactive nuclei in the sample) and after a time t, the number of remaining nuclei is N. Integration of the eq. (28.2) from t=0 to time t : N dN t dt N0 N 0 ln N NN t 0 t 0 N ln λt N0 N N 0 e λt Exponential law of radioactive decay (28.3) 14 The number of nuclei N as function of time t N N 0e λt Half-life is the time required for the number of radioactive nuclei to decrease to half the original number (No) 15 From N N 0 e t No When t T1 and N 2 2 Hence T1 N0 N0 e 2 2 T 1 1 e 2 2 T1 2e 2 ln 2 ln e T 1 2 ln 2 0.693 T1 λ λ 2 16 The units of the half-life are second (s), minute (min), hour (hr), day (d) and year (y). Its unit depend on the unit of decay constant. Table 28.2 shows the value of half-life for several isotopes. Isotope Half-life 238 92 U 226 88 Ra 4.5 109 years 210 884 Po 234 90Th 222 86 Rn 138 days 214 83 Bi 20 minutes 1.6 103 years 24 days 3.8 days Table 28.2 17 28.1.6 Activity of radioactive sample (A) dN of a radioactive sample. dt is defined as the decay rate Its unit is number of decays per second. Other units for activity are curie (Ci) and becquerel (Bq) – S.I. unit. Unit conversion: 1 Ci 3.7 1010 decays per second 1 Bq 1 decay per second Relation between activity (A) of radioactive sample and time t : dN From the law of radioactive decay : N dN dt and definition of activity : A dt 18 Thus A N and N N 0e A N 0e t t N 0 e t and A0 N 0 A A0 e Activity at time t λt (28.4) Activity at time, t =0 19 Example 28.1.1 : A radioactive nuclide A disintegrates into a stable nuclide B. The half-life of A is 5.0 days. If the initial number of nuclide A is 1.01020, calculate the number of nuclide B after 20 days. 20 Solution : T1/ 2 5.0 days; N0 1.010 ; t 20 days A BQ The decay constant is given by The number of remaining nuclide A is N N0e t The number of nuclide A that have decayed is Therefore the number of nuclide B formed is 20 Example 28.1.2 : 80% of a radioactive substance decays in 4.0 days. Determine i. the decay constant, ii. the half-life of the substance. 21 Solution : At time The number of remaining nuclei is i. By applying the exponential law of radioactive decay, thus the decay constant is t N N0e ii. The half-life of the substance is 22 Example 28.1.3 : A thorium-228 isotope which has a half-life of 1.913 years decays by emitting alpha particle into radium-224 nucleus. Calculate a. the decay constant. b. the mass of thorium-228 required to decay with activity of 12.0 Ci. c. the number of alpha particles per second for the decay of 15.0 g thorium-228. (Given the Avogadro constant, NA =6.02 1023 mol1) Solution : T1/ 2 1.913 y 1.913 365 24 60 60 6.03 107 s a. The decay constant is given by T1/ 2 ln 2 23 Solution : b. By using the unit conversion ( Ci decay/second ), 1 Ci 3.7 1010 decays per second the activity is Since A N then A N If 6.02 1023 nuclei of Th-228 has a mass of 228 g thus 3.86 1019 nuclei of Th-228 has a mass of 24 Solution : c. If 228 g of Th-228 contains of 6.02 1023 nuclei thus 15.0 g of Th-228 contains of Therefore the number of emitted alpha particles per second is given by Ignored it. dN A dt N 25 Example 28.1.4 : A sample of radioactive material has an activity of 9.00 x 10 12 Bq. The material has a half-life of 80.0 s. How long will it take for the activity to fall to 2.00 x 10 12 Bq ? Solution A A0 e λt A e t Ao ln 2 T1 λ 2 ln 2 t1/ 2 A ln Ao A t t ln Ao 174 s 26 Example 28.1.5 : N N 0e λt ln 2 t1/ 2 N = 25% , No = 100% t =34.6 min (1.72 h) 27 Learning Outcome: 28.2 Radioisotope as tracers (1/2 hour) At the end of this chapter, students should be able to: Explain the application of radioisotopes as tracers. 28 28.2 Radioisotope as tracers • Radioisotope (unstable isotope) is an isotope which is exhibits radioactivity (known as radioactive isotope). a) Blood volume • The volume of blood in the bloodstream, V2 can be determined by using dilution method as given below. A2 V2 V1 A1 A1 A2 V1 V2 29 where A1 A2 V1 V2 A1 = activity of the blood drawn from the patient A2 = activity of the blood in the bloodstream V1 = volume of the blood drawn from the patient V2 = volume of blood in the bloodstream of the patient A1 activity per unit volume of the blood draw nfrom the patient V1 A2 activity per unit volume of the blood in the blood stream V2 30 Example 28.2.1 A small volume of a solution which contains a radioactive 4 isotope of sodium Na-24 has an activity of 1.5 x 10 Bq. The solution is injected into the bloodstream of a patient. The half-life of the sodium isotope is 15 hours. After 30 3 hours, the activity of 1.0 cm of blood is measured and found to be 0.50 Bq. Estimate the volume of blood in the patient. 31 Solution 28.2.1 A2 Ao e t A2 (1.5 104 )e (ln 2 /15 )(30 ) A2 3.75103 Bq Activity per unit volume of blood in the patient Activity per unit volume of blood draw nf rom the patient A2 A1 V2 V1 3.75103 0.5 V2 1106 V2 7.5 103 m3 32 b) Detecting leaks in underground pipes. The exact position of an underground pipe can be located if a small quantity of radioactive liquid is added to the liquid being carried by the pipe. Geiger counter can be used to detect the leaks. Any leaks would be detected by an increase in radiation reading . The soil close to the leak becomes radioactive. The short-lived radioisotope is used to avoid from the permanent contamination of the soil. 33 c) Detecting brain tumors. • Technitium-99 is a gamma emitter (half-life 6 hours) and is used as a medical tracer. • When injected into the blood stream, 99 Tc will not be absorbed by the brain, because of the blood-brain barrier. • However, tumors do not have this barrier. • Thus, brain tumors readily absorb the 99 Tc. • These tumors then show as gamma-ray emitters on detectors external to the body. • The short-lived radioisotope is used so that it can quickly eliminate from the body. 34 Good luck For 2nd semester examination 35