ppt
advertisement

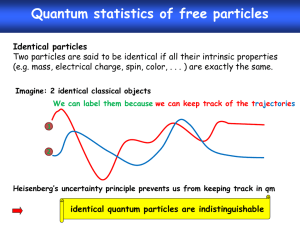
Lecture 21. Grand canonical ensemble (Ch. 7)
Boltzmann statistics (T, V, N) :
Reservoir R
U0 -
1
P i
exp i
Z T ,V ,...
System S
Plan: we want to generalize this result to the case where
both energy and particles can be exchanged with the
environment.
Reservoir
UR, NR, T,
Quantum statistics (T, V, ):
Gibbs factor
Grand part. Func.
N E
exp
k
T
B
: a (quantum) microstate
Z
System
E, N
Grand free energy
kBT ln Z
(or grand potential)
The Gibbs Factor
Reservoir
UR, NR, T,
System
E, N
1 and 2 - two microstates of
the system (characterized by the
spectrum and the number of
particles in each energy level)
R
2
1
S
the total multiplicity: Total i S i R i 1R i R i
P 2
P 1
R 2
R 1
For reservoir: dS R
exp S R 2 / k B
S R 2 S R 1
exp
k
exp S R 1 / k B
B
1
dU R PdVR dN R (thermodynamic identity)
T
neglect
The changes U and N for the reservoir = -(U and N for the system).
S R 2 S R 1
1
ES 2 ES 1 N S 2 N S 1
T
P 2 expN S 2 ES 2 / k BT
P1 expN S 1 ES 1 / k BT
Gibbs factor
N S ni ES ni Ei
i
i
N E
exp
k
T
B
The Grand Partition Function
- proportional to the probability that the
Gibbs factor
N E
exp
system in the state contains N
k
T
B
particles and has energy E
the probability that the system is in
state with energy E and N
particles:
N E
1
P exp
Z
k BT
the grand partition function or the Gibbs sum:
T ,V , N n N / V T , n
N E
Z exp
k
T
B
is the index that refers to a specific microstate of the system, which is
specified by the occupation numbers ni: s {n1, n2,.....}. The summation
consists of two parts: a sum over the particle number N and for each N, over
all microscopic states i of a system with that number of particles.
The systems in equilibrium with the reservoir that supplies both
energy and particles constitute the grand canonical ensemble.
From Particle States to Occupation Numbers
Systems with a fixed number of particles
in contact with the reservoir, occupancy
ni<<1
4
3
2
1
Systems which can exchange both
energy and particles with a reservoir,
arbitrary occupancy ni
1 N
Z1
N!
U1
ln Z1
3
U nU1
1
Z total
The energy was fluctuating, but the total
number of particles was fixed. The role of
the thermal reservoir was to fix the
mean energy of each particle (i.e., each
system). The identical systems in contact
with the reservoir constitute the canonical
ensemble. This approach works well for
the high-temperature (classical) case,
which corresponds to the occupation
numbers <<1.
4
2
nE4
N ni
i
E ni Ei
i
When the occupation numbers are ~ 1, it
is to our advantage to choose, instead of
particles, a single quantum level as the
system, with all particles that might
occupy this state. Each energy level is
considered as a sub-system in equilibrium
with the reservoir, and each level is
populated from a particle reservoir
independently of the other levels.
From Particle States to Occupation Numbers (cont.)
We will consider a system of identical non-interacting
particles at the temperature T, i is the energy of a single
particle in the i state, ni is the occupation number (the
occupancy) for this state:
N s n
i
i
The energy of the system in the state s {n1, n2, n3,.....} is:
Es n11 n2 2 n3 3 ... ni i
The grand partition function:
i
ni ni i
N s E s
ni ni i
i
i
Z exp
exp
exp
k
T
k
T
k
T
s
B
B
B
ni
ni i
ni i
Z exp
kBT
ni i
The sum is taken over all possible
occupancies and all states for each
occupancy.
Grand free energy (Landau free energy)
The grand partition function:
ni i
Z exp
k
T
ni i
B
Similar to partition function Z which is related to
Helmholtz free energy F , grand partition function Z
is related to another thermodynamic potential:
Grand free energy T ,V , kBT ln Z Pr. 7.7 (Pg. 262)
In thermodynamics (Pr. 5.23, Pg. 166)
U TS N
d SdT PdV Nd
PV
The Grand Partition Function of an Ideal Quantum Gas
ni i
ni i N ni E s
i
Z exp
i
kBT
ni i
a microstate s {n , n , n ,.....}
1
2
3
ni i
ni i
exp
exp
Zi
k BT i ni
k BT i 0
ni i
1: a, b
e.g.
2: c, d
The sum is taken over all possible values of ni
ac ad bc bd a bc d
Z Z i
i
ni i
Zi exp
kBT
ni
depending on the quantum nature of particles
The Grand Partition Function of an Ideal Quantum Gas
What is the meaning of the grand
partition function formula:
Z Z i
i
The partition function of each quantum level is
independent of other levels.
Each energy level is considered as a sub-system in
equilibrium with the reservoir, and each level is populated
from a particle reservoir independently of the other
levels.
Page 266: The “system” and the “reservoir” therefore
occupy the same physical space.
The mean occupancy at ith level
The probability of a state (ith level) to be occupied by ni quantum particles:
ni i
Gibbs factor
1
P i , ni
exp
grand partition function
Zi
k
T
B
ni ni P i , ni , sum over all possible ni .
ni i
1
ni ni
exp
Z
k
T
ni
i
B
Pr. 7.6 (Pg. 261)
1
1
exp ni i
k T Z
N B
Z i ni
ni
Z
N2
k BT
Z
2
2 Z
2
N
N kBT
1
Zi
Z i
1 ln Z i
1
k T
B
ni i
Zi exp
kBT
ni
Bosons and Fermions
One of the fundamental results of quantum mechanics is that all
particles can be classified into two groups.
Bosons: particles with zero or integer spin (in units of ħ).
Examples: photons, all nuclei with even mass numbers. The
wavefunction of a system of bosons is symmetric under the
exchange of any pair of particles: (...,Qj,...Qi,..)=
(...,Qi,...Qj,..). The number of bosons in a given state is
unlimited.
Fermions: particles with half-integer spin (e.g., electrons, all
nuclei with odd mass numbers); the wavefunction of a system of
fermions is anti-symmetric under the exchange of any pair of
particles: (...,Qj,...Qi,..)= -(...,Qi,...Qj,..). The number of
fermions in a given state is zero or one (the Pauli exclusion
principle).
Bosons and Fermions (cont.)
The Bose or Fermi character of composite objects: the
composite objects that have even number of fermions are
bosons and those containing an odd number of fermions are
themselves fermions.
(an atom of 3He = 2 electrons + 2 protons + 1 neutron hence
3He atom is a fermion)
In general, if a neutral atom contains an odd # of neutrons then
it is a fermion, and if it contains en even # of neutrons then it is
a boson.
The difference between fermions and bosons is specified by the
possible values of ni:
fermions: ni = 0 or 1
bosons: ni = 0, 1, 2, .....
Bosons and Fermions (cont.)
fermions: ni = 0 or 1
bosons: ni = 0, 1, 2, .....
distinguish.
n1
particles
n2
Bose
n1
statistics
n2
Fermi
n1
statistics
n2
1
2
1
2
3
1
3
2
3
4
1
4
2
4
3
1
1
2
2
1
3
2
3
3
1
4
2
4
3
4
1
2
1
1
2
1
2
3
2
1
3
1
3
2
3
2
3
4
3
1
4
1
4
2
4
2
4
3
4
3
Consider two noninteracting particles
in a 1D box of
length L. The total
energy is given by
En1 ,n2
h2
2
2
n
n
1
2
8m L2
The Table shows all
possible states for
the system with the
total energy
n n2 25
2
1
2
Problem (partition function, fermions)
Calculate the partition function of an ideal gas of N=3 identical
fermions in equilibrium with a thermal reservoir at temperature T.
Assume that each particle can be in one of four possible states with
energies 1, 2, 3, and 4. (Note that N is fixed).
1
1
1
1
0
2
1
0
1
1
3
1
1
0
1
4
0
1
1
1
a state with Ei
The Pauli exclusion principle leaves
only four accessible states for such
system. (The spin degeneracy is
neglected).
the number of particles in the
single-particle state
The partition function (canonical ensemble):
Z 3 exp Ei
Ei
exp 1 2 3 exp 1 3 4
exp 1 2 4 exp 2 3 4
Problem (partition function, fermions)
Calculate the grand partition function of an ideal gas of
fermions in equilibrium with a thermal and particle reservoir (T,
). Fermions can be in one of four possible states with energies
1, 2, 3, and 4. (Note that N is not fixed).
4
3
2
each level I is a sub-system independently
“filled” by the reservoir
1
Z 1 exp i
i
1 exp 1 1 exp 2 1 exp 3 1 exp 4
1 exp 1 exp 2 exp 3 exp 4
exp 2 1 2 exp 2 2 3 ...