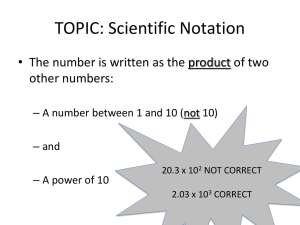
Measurement
advertisement

Chapter 2 Measurement and Problem Solving Homework Exercises (optional, in Tro textbook) 1 through 27 (odd) Problems (in Tro textbook) 29-65 (odd) 67-91 (odd) 93-99 (odd) Cumulative Problems (in Tro textbook) 101-117 (odd) Highlight Problems (optional, in Tro textbook) 119, 121 Scientific Notation: Writing Large and Small Numbers Scientific Notation A system in which an ordinary decimal number (m) is expressed as a product of a number between 1 and 10, multiplied by 10 raised to a power (n) Used to write very large or very small numbers Based on powers of 10 m 10 n Scientific Notation: Writing Large and Small Numbers Consists of a number (coefficient) followed by a power of 10 (x 10n) 7.03 10 Coefficient or decimal part 2 exponent exponential term or part Negative exponent: Number is less than 1 Positive exponent: Number is greater than 1 Scientific Notation: Writing Large and Small Numbers In an ordinary cup of water there are: 7,910,000,000,000,000,000,000,000 molecules Each molecule has a mass of: 0.0000000000000000000000299 gram In scientific notation: 7.91 х 1024 molecules 2.99 х 10-23 gram To Express a Number in Scientific Notation: For small numbers (<1): 1) Locate the decimal point 2) Move the decimal point to the right to give a number (coefficient) between 1 and 10 3) Write the new number multiplied by 10 raised to the “nth power” where “n” is the number of places you moved the decimal point. It has a negative sign If the decimal point is moved to the right, then the exponent is a negative number (× 10-n) To Express a Number in Scientific For large numbers (>1): 1) Locate the decimal point 2) Move the decimal point to the left to give a number (coefficient) between 1 and 10 3) Write the new number multiplied by 10 raised to the “nth power” where “n” is the number of places you moved the decimal point. It has a positive sign. If the decimal point is moved to the left, the exponent is a positive number (× 10n) Examples Write each of the following in scientific notation 12,500 0.0202 37,400,000 0.0000104 Examples 12,500 Decimal place is at the far right Move the decimal place to a position between the 1 and 2 Coefficient (1.25) The decimal place was moved 4 places to the left (large number) so exponent is positive 1.25x104 Examples 0.0202 Move the decimal place to a position between the 2 and 0 Coefficient (2.02) The decimal place was moved 2 places to the right (small number) so exponent is negative 2.02x10-2 Examples 37,400,000 Decimal place is at the far right Move the decimal place to a position between the 3 and 7 Coefficient (3.74) The decimal place was moved 7 places to the left (large number) so exponent is positive 3.74x107 Examples 0.0000104 Move the decimal place to a position between the 1 and 0 Coefficient (1.04) The decimal place was moved 5 places to the right (small number) so exponent is negative 1.04x10-5 Scientific Notation and Calculators 1) Enter the coefficient (number) 2) Push the key: EE or EXP Then enter only the power of 10 3) If the exponent is negative, use the key: (+/-) 4) DO NOT use the multiplication key: X to express a number in sci. notation Converting Back to a Standard Number 1) Determine the sign of the exponent, n If n is + the decimal point will move to the right (gives a number greater than one) If n is – the decimal point will move to the left (gives a number less than one) 2) Determine the value of the exponent of 10 The “power of ten” determines the number of places to move the decimal point Using Scientific Notation To compare numbers written in scientific notation First compare the exponents of 10 The larger the exponent, the larger the number If the exponents are the same, then compare coefficients directly Which number is larger? 21.8 х 103 or 2.05 х 104 2.18 х 104 > 2.05 х 104 Significant Figures: Writing Numbers to Reflect Precision Two kinds of numbers Counted (exact) Measured Measured Numbers Scientific numbers are reported so that all digits are certain except the last digit which is estimated A measurement: involves reading a measuring device always has some amount of uncertainty uncertainty comes from the tool used for comparison e.g. some rulers show smaller divisions (more precise) than others Measured Numbers Always have to estimate the value between the two smallest divisions on a measuring device Every person will estimate it slightly differently, so there is some uncertainty present as to the true value 2.8 cm 2.8 to 2.9 cm 2.9 cm Significant Figures: Writing Numbers to Reflect Precision Scientific numbers are reported so every digit is certain except the last which is estimated To indicate the uncertainty of a single measurement scientists use a system called significant figures Significant figures: All digits known with certainty plus one digit that is uncertain Counting Significant Figures The last digit written in a measurement is the number that is considered to be uncertain (estimated) Unless stated otherwise, the uncertainty in the last digit is ±1 The precision of a measured quantity is determined by number of sig. figures A zero in a measurement may or may not be significant significant zeros place-holding zeros (not significant) Counting Significant Figures Nonzero integers are always significant Zeros (may or may not be significant) It is determined by its position in a sequence of digits in a measurement Leading zeros never count as significant figures Captive (interior) zeros are always significant Trailing zeros are significant if the number has a decimal point Exact Numbers Exact numbers occur in definitions or in counting Numbers known with no uncertainty Unlimited number of significant figures (never limit the no. of sig. figures in a calculation) They are either Counting numbers 7 pennies, 6 pills, 4 chairs Defined numbers 12 in = 1 ft 1 gal = 4 quarts 1 minute = 60 seconds Significant Figures in Calculations Calculations cannot improve the precision of experimental measurements The number of significant figures in any mathematical calculation is limited by the least precise measurement used in the calculation Two operational rules to ensure no increase in measurement precision addition and subtraction multiplication and division Significant Figures in Calculations: Multiplication and Division Product or quotient has the same number of significant figures as the factor with the fewest significant figures Count the number of significant figures in each number. The least precise factor (number) has the fewest significant figures Rounding Round the result so it has the same number of significant figures as the number with the fewest significant figures Rounding To round the result to the correct number of significant figures If the last (leftmost) digit to be removed: • is less than 5, the preceding digit stays the same (rounding down) • is equal to or greater than 5, the preceding digit is rounded up • In multiple step calculations, carry the extra digits to the final result and then round off Multiplication/Division Example: 5 SF 4 SF 3 SF 0.1021 0.082103 273 1.1 2.1 2.080438 2 SF 2 SF The number with the fewest significant figures is 1.1 so the answer has 2 significant figures Multiplication/Division Example: 4 SF 5 SF 3 SF 3 SF 0.1021 × 0.082103 × 273 = 2.288481 2.29 The number with the fewest significant figures is 273 so the answer has 3 significant figures Significant Figures in Calculations: Addition and Subtraction Sum or difference is limited by the quantity with the smallest number of decimal places Find quantity with the fewest decimal places Round answer to the same decimal place Addition/Subtraction Example: 1 d.p. 3 d.p. 2 d.p. 171 .5 72 .915 8 .23 236 .185 236.2 1 d.p. The number with the fewest decimal places is 171.5 so the answer should have 1 decimal place Measurement The most useful tool of the chemist Most of the basic concepts of chemistry were obtained through data compiled by taking measurements How much…? How long…? How many...? These questions cannot be answered without taking measurements The concepts of chemistry were discovered as data was collected and subjected to the scientific method Measurement The estimation of the magnitude of an object relative to a unit of measurement Involves a measuring device e.g. meter stick, scale, thermometer The device is calibrated to compare the object to some standard (inch/centimeter, pound/kilogram) Quantitative observation with two parts: A number and a unit Number tells the total of the quantity measured Unit tells the scale (dimensions) Measurement A unit is a standard (accepted) quantity Describes what is being added up Units are essential to a measurement For example, you need “six of sugar” teaspoons? ounces? cups? pounds? The Basic Units of Measurement Units tells the magnitude of the standard Two most commonly used systems of units of measurement U.S. (English) system: Used in everyday commerce (USA and Britain*) Metric system: Used in everyday commerce and science (The rest of the world) SI Units (1960): A modern, revised form of the metric system set up to create uniformity of units used worldwide (world’s most widely used) The Standard Units: The Metric/SI System A decimal system of measurement based on the meter and the gram It has a single base unit per physical quantity All other units are multiples of 10 of the base unit The power (multiple) of 10 is indicated by a prefix The Standard Units: The Metric System In the metric system there is one base unit for each type of measurement length volume mass The base units multiplied by the appropriate power of 10 form smaller or larger units The prefixes are always the same, regardless of the base unit milligrams and milliliters both mean 1/1000 of the base unit The Standard Units: Length Meter Base unit of length in metric and SI system About 3 ½ inches longer than a yard 1 m = 1.094 yd The Standard Units: Length Other units of length are derived from the meter Commonly use centimeters (cm) 1 m = 100 cm 1 inch = 2.54 cm (exactly) The Standard Units: Volume Measure of the amount of three-dimensional space occupied by a object Derived from length SI unit = cubic meter (m3) Metric unit = liter (L) or 10 cm3 Commonly measure smaller volumes in cubic centimeters (cm3) Volume = side × side × side Volume = side × side × side The Standard Units: Volume Since it is a threedimensional measure, its units have been cubed SI base unit = cubic meter (m3) This unit is too large for practical use in chemistry Take a volume 1000 times smaller than the cubic meter, 1dm3 The Standard Units: Volume Metric base unit = 1dm3 = liter (L) 1L = 1.057 qt Commonly measure smaller volumes in cubic centimeters (cm3) Take a volume 1000 times smaller than the cubic decimeter, 1cm3 The Standard Units: Volume Metric base unit = 1dm3 = liter (L) 1L = 1.057 qt Commonly measure smaller volumes in cubic centimeters (cm3) Take a volume 1000 times smaller than the cubic decimeter, 1cm3 The Standard Units: Volume The most commonly used unit of volume in the laboratory: milliliter (mL) 1 mL = 1 cm3 1 L= 1 dm3 = 1000 mL 1 m3 = 1000 dm3 = 1,000,000 cm3 Use a graduated cylinder or a pipette to measure liquids in the lab The Standard Units: Mass Measure of the total quantity of matter present in an object SI unit (base) = kilogram (kg) Metric unit (base) = gram (g) Commonly measure mass in grams (g) or milligrams (mg) 1 kg = 1000 g 1 g = 1000 mg 1 kg = 2.205 pounds 1 lb = 453.6 g Prefixes Multipliers One base unit for each type of measurement Length (meter), volume (liter), and mass (gram*) The base units are then multiplied by the appropriate power of 10 to form larger or smaller units base unit = meter, liter, or gram Prefixes Multipliers (memorize) × base unit Mega Kilo Base Deci Centi Milli Micro Nano (M) 1,000,000 (k) 1,000 1 meter liter gram (d) 0.1 (c) 0.01 (m) 0.001 (µ) 0.000001 (n) 0.000000001 106 103 100 10-1 10-2 10-3 10-6 10-9 Prefix Multipliers For a particular measurement: Choose the prefix which is similar in size to the quantity being measured Keep in mind which unit is larger e.g. A kilogram is larger than a gram, so there must be a certain number of grams in one kilogram Choose the prefix most convenient for a particular measurement n < µ < m < c < base < k < M Converting from One Unit to Another: Equalities A fixed relationship between two quantities Shows the relationship between two units that measure the same quantity The relationships are exact, not measured 1 min = 60 s 12 inches = 1 ft 1 dozen = 12 items (units) 1L = 1000 mL 16 oz = 1 lb 4 quarts = 1 gallon Converting from One Unit to Another: Conversion Factors Many problems in chemistry involve a conversion of units Conversion factor: An equality expressed as a fraction Used as a multiplier to convert a quantity in one unit to its equivalent in another unit May be exact or measured Both parts of the conversion factor should have the same number of significant figures Solving Multistep Conversion Problems: Dimensional Analysis Example (Conversion Factors Stated within a Problem) The average person in the U.S. consumes one-half pound of sugar per day. How many pounds of sugar would be consumed in one year? 1) State the initial quantity given (+unit): One year State the final quantity to find (+unit): Pounds 2) Write a sequence of units (map) which begins with the initial unit and ends with the desired unit: year day pounds 1 cal 4.184 J Solving Multistep Conversion Problems: Dimensional Analysis Example 3) For each unit change, State the equalities: Every equality will have two conversion factors 365 days = 1 year 0.5 lb sugar =1day year day pounds Solving Multistep Conversion Problems: Dimensional Analysis Example State the conversion factors: 0.5 lb. sugar 1day and 1 day 0.5 lb. sugar 4) Set Up the problem: 1 year 365 day(s) 1 year 0.5 lb sugar 1 day 183 lbs. sugar Guide to Problem Solving when Working Dimensional Analysis Problems Identify the known or given quantity and the units of the new quantity to be determined Write out a sequence of units which starts with your initial units and ends with the desired units (“solution map”) Write out the necessary equalities and conversion factors Perform the mathematical operations that connect the units Check that the units cancel properly to obtain the desired unit Does the answer make sense? Density The ratio of the mass of an object to the volume occupied by that object Tells how tightly the matter within an object is packed together Units for solids and liquids = g/cm3 1 cm3 = 1 mL so can also use g/mL Unit for gases = g/L Density: solids > liquids >>> gases Density mass volume m d v Density Can use density as a conversion factor between mass and volume Density of some common substances given in Table 2.4, page 33 You will be given any densities on tests EXCEPT water Density of water is 1.0 g/cm3 at room temperature 1.0 mL of water weighs how much? How many mL of water weigh 15 g? Density To determine the density of an object Use a scale to determine the mass Determine the volume of the object Calculate it if possible (cube shaped) Can also calculate volume by determining what volume of water is displaced by an object Volume of Water Displaced = Volume of Object Density Problem Iron has a density of 7.87 g/cm3. If 52.4 g of iron is added to 75.0 mL of water in a graduated cylinder, to what volume reading will the water level in the cylinder rise? Vf ? m 52.4 g d 7.87 g cm Vi 7 5 .0 mL 3 Density Problem Solve for volume of iron density mass volume 52.4 g iron volume mass density 1 mL iron 7.87 g iron 1 cm 3 = 1 mL 6 .658 mL iron 6 .658 mL iron + 75.0 mL water = 8 1.7 mL total End