0 -1 e
advertisement

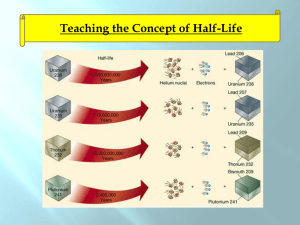
Nuclear Chemistry Chapter 21 Stable vs. Unstable Nuclei 1. Most nuclei are stable – do not change 2. Some nuclei are unstable (radioactive) • • • Change into a different nucleus Spontaneous process – happens naturally, by itself Releases radiation Only nuclear reactions can change a nucleus. No chemical process can Radium Radon + Radiation 1. The radium was unstable (radioactive) 2. Turned into a different element (decayed) 3. The lost mass was turned into radiation Nuclear Radiation • Is spontaneously emitted from a radioactive nucleus • Can not be seen, smelled, heard • Can be detected using a Geiger counter or photographic film Uses of Radiation 1. 2. 3. 4. 5. 6. Nuclear fuel (235U and 239Pu) Nuclear Weapons Irradiated Food Smoke Alarms (Amercium-241) Cancer treatment (Cobalt-60) Medical Tracers Types of Nuclear Radiation Alpha particle Helium nucleus 2 p+ a (42He) Beta particle b (0-1e) Gamma rays g (00g) 2n fast-moving electron. e- high energy form of electromagnetic radiation The Electromagnetic Spectrum Light Dangerous (ionizing) Safe radiation (non-ionizing) Radio Radar Micro IR Visible Light UV Xrays Gamma Produced by nuclear decay What Stops Radiation Paper Alpha (a) Beta (b) Gamma (g) Al Foil Lead. Wood Iron, Concrete Decay Equations Alpha Decay 238 U 4 He + 92 2 Beta Decay 234 Th 0 e + 90 -1 234 234 90Th 91Pa Decay Equations Gamma Decay Occurs with alpha and beta decay No change in atomic mass (gamma radiation has no mass 00g) Decay: Ex 1 What product is formed when radium-226 undergoes alpha decay? 226 88U 4 2He 226 88U 4 2He + + ? 222 86Rn Decay: Ex 2 What element undergoes alpha decay to form lead-208? ? 4 212 84Po 4 2He + 2He + 208 82Pb 208 82Pb Decay: Ex 3 What isotope is produced when thorium-231 beta decays? 231 90Th 0 -1e 231 90Th 0 -1e + + ? 231 91Pa Positron Emission – Same mass an electron, but opposite charge – Form of anti-matter 0 e 1 Electron Capture – Nucleus captures a core electron – electron is added rather than lost Common Particles Particle Symbol Apha 4 He 2 Beta 0 e -1 Positron 0 e 1 Electron 0 e -1 Proton Neutron 1 H 1 or 11p 1 n 0 Decay: Ex 4 Write the equation that describes oxygen-15 undergoing positron emission. Write the equation that describes mercury-201 undergoing electron capture Which nuclei are radioactive (unstable) 1. All elements have at least one radioactive isotope 2. All isotopes of elements heavier than Lead (element 82) are radioactive 3. All elements heavier than 92 (U) are manmade and radioactive 82 Pb At least one radioactive isotope 207.2 All isotopes are radioactive • Belt of stability – based on neutron:proton ratio – Below ~20 = 1:1 ratio stable – Ratio increases with increasing # protons – Isotopes outside the belt try to decay and get on the belt Decay Modes • Above belt – Too many neutrons – Beta emission • Below belt – Too few neutrons – electron capture or positron emission • Atomic # >84 – Alpha Decay • Most heavy isotopes (above 84) decay by alpha emission • Slide down to lead206 Decay Modes: Ex 1 Predict the decay mode for carbon-14 Too many n’s, prefers 1:1 8n : 6p 14 0 e + 14 N C 6 -1 7 (ratio now 1:1) Decay Modes: Ex 2 Predict the decay mode for xenon-118 64n : 54p =1.2 Too few n’s (check graph) 0 e 118 I Xe + 54 -1 53 or 118 Xe 0 e + 118 I 54 -1 53 118 Decay Modes: Ex 3 Predict the decay mode for plutonium-239 Predict the decay mode for indium-120 Further Observations • Magic #’s - Nuclei with 2, 8, 20, 28, 50 or 82 protons or 2, 8, 20, 28, 50 or 126 neutrons are especially stable. • Nuclei with even #s of both protons and neutrons are more stable than those with odds numbers. Ex: 63Cu and 65Cu are abundant, but 64Cu is not. Why? Transmutation • Rutherford(1919) – First successful alchemist 14 N + 4 He 17 O + 1 H 7 2 8 1 14 N(a,p) 17 O 7 8 • Modern methods – Particle Accelerators (Cyclotrons) – Use neutrons or other elements (creation of transuranium elements) Transmutation: Ex 1 Write the balanced nuclear equations for the process : 2713Al(n, a) 2411Na Transmutation: Ex 2 Write the shorthard notation for: 16 1 H O + 8 1 13 4 He N + 7 2 Transmutation: Neutrons • Neutrons produced from radioactive decay • Cobalt-60 is used in radiation therapy 58 Fe 26 59 Fe 26 59 Co 27 + 10n + 10n 59 Fe 26 59 Co 27 60 Co 27 + 0-1e Transmutation: Transuranium Elements 23992U 23993Np + 0-1e 238 1 n U + 92 0 230 4 He 242 Cm + 1 n Pu + 94 2 96 0 209 64 Ni 272 1 n Bi + Uuu + 83 28 111 0 Half-Life • Half-life - The time during which one-half of a radioactive sample decays – Ranges from fraction of a second to billions of years. – You can’t hurry half-life. Carbon-14 dating • 14C atoms get incorporated into living things through breathing and eating. • Ratio of 14C to12C in a living organism is equal to that in the atmosphere • When an organism dies, the ratio changes as 14C radioactively decays. The amount of 14C starts to decrease over time. Carbon-14 dating • By measuring the amount of 14C in traces of onceliving organisms, one can determine how long ago it died. – E.g., a 5730 years after death, only half of the 14C remains. • Reasonable to up to 50,000 years. • There is a 15% margin of error • Used in mummies, the Dead Sea Scrolls, Shroud of Turin Half-Life Isotope Uranium-238 Half-life 4.51x109 years Lead-210 20.4 years Polonium-214 1.6x10-4 seconds The polonium-214 will decay much sooner than the uranium. The uranium will be radioactive pretty much until the earth is destroyed when our sun goes out in 10 billion years. Half-life: Example 1 Carbon-14 has a half-life of 5730 years and is used to date artifacts. How much of a 26 g sample will exist after 3 half-lives? How long is that? Half-life: Example 1 # of half-lives # of Years passed 0 0 1 5730 2 3 Amount of Carbon14 remaining 26 grams Half-life: Example 2 Tritium undergoes beta decay and has a half life of 12.33 years. How much of a 3.0 g sample of tritium remains after 2 half-lives? Solution to Problem # of halflives 0 1 2 # of Years passed 0 Amount of tritium remaining Half-life: Example 3 Radon-226 has a half-life of 1600 years? How much of a 30 gram sample remains after 6400 years? Solution to Problem # of halflives 0 1 2 3 4 5 # of Years passed 0 Amount of radon remaining Half-life: Example 4 Cesium-137 has a half-life of 30 years. If you start with a 200 gram sample, and you now have 25 grams left, how much time has passed? Solution to Problem # of halflives 0 1 2 3 4 5 # of Years passed 0 Amount of Cesium remaining Half-life: Example 5 Calcium-45 has a half-life of 160 days. If you start with a 500 gram sample, and you now have 31.25 grams left, how much time has passed? Solution to Problem # of halflives 0 1 2 # of Years passed 0 Amount of Calcium remaining Rate Law First order rate law Rate = kN (N is the initial concentration) Rate = -DN = dN = -kN Dt dt dN = -kN dt dN = -kdt N ∫dN = ∫-kdt N ∫dN = -k∫dt N lnNt = -kt N0 (Integrate left from N0 to Nt and time from 0 to t) Calculating k or the half-life lnNt = -kt N0 ln1 = -kt½ 2 k = 0.693 t½ Rate Law: Ex 1 Uranium-238 has a half-life of 4.5 X 109 yr. If 1.000 mg of a 1.257 mg sample of uranium238 remains, how old is the sample? k = 0.693 t½ k = 0.693 4.5 X 109 yr = 1.5 x10-10 yr lnNt = -kt N0 ln 1.000 = -(1.5 x10-10 yr)t 1.257 t = 1.7 X 109yr Rate Law: Ex 2 A wooden object is found to have a carbon-14 activity of 11.6 disintegrations per second. Fresh wood has 15.2 disintegrations per second. If the half-life of 14C is 5715 yr, how old is the object? Rate Law: Ex 2 A wooden object is found to have a carbon-14 activity of 11.6 disintegrations per second. Fresh wood has 15.2 disintegrations per second. If the half-life of 14C is 5715 yr, how old is the object? ANS: 2230 yr Rate Law: Ex 3 After 2.00 yr, 0.953 g of a 1.000 g sample of strontium-90 remains. How much remains after 5.00 years? lnNt = -kt N0 ln0.953 = -k(2.00 yr) 1.000 k = 0.0241 yr-1 lnNt = -kt N0 ln x = (0.0241 yr-1)(5.00 yr) 1.000 ln x = -0.120 1.000 x = e-0.120 x =0.887 g Ex 4 A sample for medical imaging contains 18 F (1/2 life = 110 minutes). What percentage of the original sample remains after 300 minutes? ANS: 15.1% E = mc2 • Energy changes in chemical reactions – Exothermic – gives off energy, products mass less than reactants – Endothermic – absorbs energy, products mass more than reactants – THESE MASS CHANGES ARE WAY TOO SMALL TO MEASURE • Energy Changes in nuclear decay – Mass loss from nuclei – Energy always released – This energy is additional kinetic energy given to the products (products move faster than reactants) c = 3.00 X 108 m/s E = mc2: Ex1 92U 238.0003 amu 238.0003 amu 238 234 4 He Th + 90 2 233.9942 amu 4.0015amu 237.9957 amu Dm = -0.0046 g/mol = -4.6 X 10-6 kg/mol E = mc2 E = (4.6 X 10-6 kg/mol)(3.00X108 m/s)2 E = 4.1 X 1011 J/mol (can power a 60-W light bulb for 217 years) E = mc2: Ex 2 Calculate the energy released from the following decay. 60 Co 0 e + 60 Ni 27 -1 28 60 Co 27 0 e -1 60 Ni 28 59.933819 amu 0.00054858 amu 59.930788 amu ANS: 2.724 X 1011 J/mol E = mc2: Ex 3 The following decay produces 2.87 X 1011 J/mol of 116C. What is the mass change in this decay? 11 6C 11 5B + ANS: -3.19 X 10-3 g/mol 0 1e Binding Energy • The mass of nuclei are ALWAYS less than the masses of individual protons and neutrons (nucleons). • Mass defect • Nuclear Binding Energy – energy needed to separate nucleus into p & n – The larger the binding energy, the more stable the isotope – Iron-56 has the highest binding energy – Stars only make up to Iron-56 (unless supernova) The Four Forces Force Range Description Strong Nuclear Force Short Range (nucleus) Strongest, holds nucleus together (gluons) Electromagnetic Infinite Range Between positive and negative charges (virtual photons) Weak Nuclear Force Short Range (nucleus) Involved in some nuclear decay (quark to quark transmutations, J particle) Gravity Infinite Range Weakest, between any object with mass, even dark matter (gravitons) Strong Nuclear Force • Strong Nuclear Force – Short-range force – operates only within nuclear distances – Force between p and n that overcomes protonto-proton repulsion Binding Energy: Ex 1 Calculate the binding energy for a helium-4 nucleus given the following information: 4 2He proton neutron 4.00150 amu 1.00728 amu 1.00866 amu Mass of individual nucleons protons 2(1.00728 amu) neutrons 2(1.00866 amu) total 2.01456 amu 2.01732 amu 4.03188 amu Mass defect 4.03188 amu -4.00150 amu 0.03038 amu Mass defect = 0.03038 g/mol 0.03038 g 1 kg 1 mol 1mol 1000 g 6.022X1023 atoms = 5.045 X 10-29 kg/atom E=mc2 E = (5.045 X 10-29 kg/atom)(3.00 X 108 m/s)2 E = 4.534 X10-12 J/atom or E = 4.534 X 10-12J/ 4 nucleons E = 1.13X10-12 J/nucleon Binding Energy: Ex 2 Calculate the binding energy for an iron-56 nucleus given the following information: 56 26Fe proton neutron 55.92068 amu 1.00728 amu 1.00866 amu ANS: 1.41 X 10-12 J/nucleon Fission: Chain Reaction • Must absorb some of those neutrons or fission continues unchecked (explosion?) Turbine Moderator (water) Control Rods Uranium Fuel Rods Steam Nuclear Fission Power • Uses 235U • First commercial nuclear power - 1957 at Shippingport, PA • People living near a nuclear power plant = 1/10 radiation of a coast-to-coast jet plane trip (cosmic radiation). • Three-Mile Island (1979) - partial meltdown due. No fatalities, no serious release of radiation. • Chernobyl, Ukraine (1986) – full meltdown. 31 deaths, 260,000 exposed to high levels of radiation. Nuclear Fission: Bombs • Nuclear bombs (uranium or plutonium) • Critical Mass – minimum mass required for a chain reaction – Subcritical mass – Supercritical mass Fusion • Fusion: Combining 2 nuclei of lighter element • Thermonuclear fusion occurs at high temperatures like in the sun (3 to 40 million K). – 657 million tons of hydrogen is fused to 653 million tons of helium each second – Energy released = sunlight • Not yet feasible for commercial reactors Sources of Exposure to Radiation Natural Exposure (~80%) 1. The atmosphere (Radon and carbon-14) 2. Particles that come from outer space 3. Rocks, soil and bricks (Uranium and Thorium) 4. Foods (carbon-14) Technological Sources (~20%) 1. Nuclear weapons testing 2. High-altitude plane flights 3. X-rays (even though they are not alpha, beta or gamma) 4. Fossil fuel and nuclear electrical generation 5. Disturbances in rocks from mining, building 6. Smoking (VERY high levels) Measuring Exposure to Radiation 1. Units rad – total exposure rem – [roentgen equivalent man] – total damaging exposure millirem (mrem) – 1/1000th of a rem 2. mrem is the unit used to measure possible damage to human tissue. 3. U.S. Average = 360 mrem/year Ionizing Radiation • UV light and X-rays a, b and g from nuclear decay • Produces “free radicals” • Affects bone marrow, blood, lymph nodes Danger of Radon 1. Radon-222 gas passes in and out of the lungs. 2. Produced by decay of radium-226 from rocks, soil, and building materials. 3. Radon has a half-life of 3.825 days and decays into solid polonium-218. 4. Polonium-218 emits alpha particles which can damage lung tissue. 222 86Rn 218 218 84Po 214 84Po + 4 82Pb + 4 2He 2He