Significant Figures
advertisement

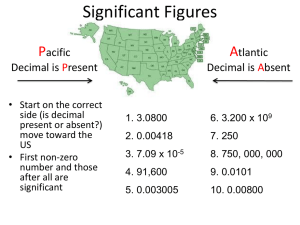
Significant Figures Warm - Up • How do you use a fire extinguisher? • What should you do if you get a chemical in your eyes? On your clothes? • What should you do if you find or break glassware in the lab? • What is the role of the fire blanket in an emergency situation? • What is the difference between a qualitative and a quantitative observation? Objective • Today I will be able to: • Successfully identify lab equipment and safety rules on a quiz • Determine the number of significant figures in a measurement • Apply significant figures to solving problems Homework • Significant Figure Practice • Dimensional Analysis Practice Agenda • Warm-Up • Quiz • Significant Figures Notes • Significant Figures Practice Significant Figures AP Chemistry Special! Significant Figures • Measurements are frequently combined • Uncertainty of the separate measurements must be reflected in your final answer, which is done by keeping track of the significant figures in each separate measurement Significant Figures •Certain numbers and the estimated digit of a measurement - Ex: 31.7 million has 3 sig figs – two (3 and 1) are certain, and one (7) is estimated Atlantic Pacific Rule Atlantic Pacific Rule • If a decimal point is Present as in 52.3 km, count from the “Pacific Side” from the first nonzero digit to the end. Meaning, count from the left side of the number - 52.3 has 3 sig figs - How many sig figs in .0093077 - There are 5 sig figs (start counting at 9) Atlantic Pacific Rule • If the decimal point is Absent, as in 1530 g, count from the Atlantic Side beginning with the first nonzero digit and going to the end, counting any zero as significant. This means start from the right - 1530 g has 3 sig figs • How many sig figs in 190,542,100ml - There are 7 sig figs Examples • .0026702 m -5 • 19.0750 kg -6 • 25,000,000,000 mm -2 • 1,908,150 L -6 • 520 ml -2 • .0102 ns -3 Sig Fig Calculations • You cannot be more precise than your least precise measurement • In multiplication and division, the measurement with the smallest number of sig digits determines how many digits are allowed in the final answer • If you have several steps, carry the extra digits. Only the final answer is rounded Examples •6.15 m x 4.026 m = ? - 6.15 m has 3 sig figs - 4.026 m has 4 sig figs - Your answer can only have 3 sig figs - 24.7599 m2 24.8 m2 Examples •.03287 g x 45.2 g = ? - .03287 g has 4 sig figs - 45.2 g has 3 sig figs - Your answer can only have three sig figs - 1.485724 g 1.49 g Sig Fig Calculations • When adding or subtracting, the number of decimal places in the answer should be equal to the number of decimal places in the number with the FEWEST places. • 0.12 + 1.6 + 10.976 = 12.696 • 12.696 12.7 (a number with one decimal place, because 1.6 has only one decimal place) Whats the SIGnificance • Swimming vs. Diving/ Gymnastics Final Note • When doing calculations with significant figures, conversion factors do not figure in • Counts and defined numbers are EXACT and have no uncertain digits • Example: if you say there are 6 people in your family it is a counted number and is considered certain. There are not 6.1 people in your family • Example: 12 inches = 1 foot is defined – do not use significant figures. 1 foot will never be 11.99 inches. In both cases, significant figures do not apply •Why are Significant Figures Significant? Exit Ticket