CHAPTER 2. SCIENTIFIC MEASUREMENTS

CHAPTER 2. SCIENTIFIC
MEASUREMENTS
CHM130
GCC Chemistry Department
Read all sections of Ch. 2
• These slide presentation are not online.
• The online NOTES contain basically the same information, just in an outline form so it takes less paper. You should print them out and bring to class
• You should take your OWN additional notes during lecture in the margins or back of pages of the online notes for anything not in the online notes.
• I’ll help you know what to write down.
• Examples are different between the online notes and slides to give you MORE examples. You should write down the examples we do in class, and then do the examples in the notes at home.
2.1 Measurements
• Measurement – number with unit
– We’ll mostly use metric units
• All measurements have uncertainty
– Sometimes you have to estimate a reading
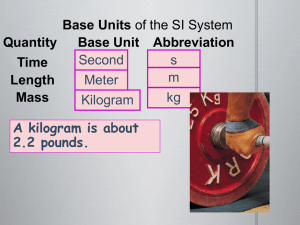
Length
• Measured in meters (cm, mm, m)
• Measured with rulers
– Some rulers have more marks than others
– Which ruler is more accurate?
– Which ruler has more uncertainty?
Mass
• Measured in grams (mg, kg, g)
• Measured by a balance
• Always write down all the numbers on a digital balance in lab!
Mass vs. Weight
Mass : the amount of matter in an object; mass is not affected by gravity.
Weight : a measure of the force of gravity.
• Mass is same anywhere, but weight differs.
Ex: An astronaut weighs 170 lbs on earth, but
29 lbs on the moon.
Volume: amount of space occupied.
• Volume is measured using beakers, flasks, graduated cylinders, syringes, burettes and pipettes.
• Units are liters (L, kL, mL)
Can you name these for fun?
What kind of unit do syringes have in hospitals? cc
What does that stand for?
Cubic centimeter
Remember this: 1 mL = 1 cm 3 = 1 cc
Remember these from the English system?
1 gallon = 4 quarts,
1 pint = 2 cups
1 quart = 2 pints
2.2 Significant Digits or Significant
Figures (Sig figs)
Significant Figures: All digits (numbers) in a measurement that are known plus one more that is estimated - a guess.
Ex: Bathroom scale vs. surgical scale – which is more accurate? Which has more sig fig?
What is the length of the candy?
Counting Significant Figures
1. Numbers 1-9 always count.
4895.2 has 5 sig figs
2. Zeroes in front never count.
0.0005454 has 4 sig figs
3. Zeroes after decimal point AND a # count.
0.0880 has 3 sig figs
28500 has 3 sig figs
4. Zeros between sig digits count.
3050 has 3 sig fig
0.002001 has 4 sig fig
Exact Number : something counted or a definition, not something measured
Exact: 14 people, 3 feet per yard, 100 cents per dollar, 5 ipods,
12 cans of beer
Not exact: 7 inches, 200 pounds, 10 ounces
As you will see, sig fig rules don’t apply to exact numbers.
How many sig figs in:
5.05
1200
0.02020
0.0005
50
50.00
123.45
8090
5
3
1
4
3
2
4
1
2.3 Rounding
Rules for rounding numbers:
1.
< 5, don’t round up.
2.
≥ 5, round up.
3. Don't change the magnitude of the number.
Holy crap what is magnitude?
How big or small the number is. Like is it in the thousands? Hundreds? Tenths? Billions? If a number is in the thousands, when you round it must STILL be in the thousands.
Round these numbers off to 3 significant figures.
1) 1.8374 1.84
2) $7162.32
3) 0.00131154
$7160
NOT 716. Seven thousand dollars is not the same as seven hundred dollars!!! (Magnitude)
0.00131
4) 24,925
24,900
2.4 Adding and Subtracting
Addition and subtraction : Your final answer must have the same decimal places as the fewest decimal places .
(Your answer can only be as accurate as the weakest link)
13.5478
- 11.20
2.3478
Final answer = 2.35
• Rounded to 2.35 since 11.20 has two decimal places
Focus on Decimal Places
2.5 Multiplication and Division
• Your final answer has the same # sig dig as the LEAST sig dig.
3.546 x 1.4 =
4.9644 = 5.0
2 sig fig cause 1.4 is 2 sig fig
Focus on Sig Fig
2.6 and 2.7 Exponential Numbers and
Scientific Notation
• Convenient method for expressing very large or very small numbers.
Writing Numbers in Scientific Notation:
Left for large numbers
Distance from the earth to the sun ~
93,000,000 miles
We need to move the decimal 7 places to the left
= 9.3 x 10 7 miles
We must have one digit before the decimal place only.
93 x 10 6 is wrong
Writing Numbers in Scientific Notation:
Right for small numbers
Radius of a carbon atom ~
0.00000000017 meters
We need to move the decimal 10 places to the right
= 1.7 x 10 -10 meters
Convert the following to scientific notation:
1. 548.005
2. 68,100,000
3. 0.000400
4. 2000
5.48005 x 10 2
6.81 x 10 7
4.00 x 10 -4
2 x 10 3
Note – Sci notation always shows sig dig’s.
The number 235,000,000 in scientific notation on your calculator: is 2.35 x 10 8
Punch in as 2.35 EE 8
DO NOT
type in “x10
^”. The EE or EXP is in place of the x10^
Calculator
• You must have your own scientific calculator
• Your instructor will show you examples
• You may not use graphing calculators on quizzes or exams – Department rules
• You may not use your phone as a calculator on quizzes or exams either!
Practice Problem
• Try this on your calculator – write in your notes
• 2.84 x 10 23 / 7.24 x 10 12 = ?
3.92 x 10 10
If you got this wrong, you forgot to use your EE or
EXP button correctly. You MUST be able to do problems like this on your calculator. Raise your hand if you need help or see me after class.
Making Sci notation show
• What if your calculator shows you a large number like 123456789? You don’t want to have to count to figure out the scientific notation. On most calculators push “2 nd ”
“SCI” and then it shows 1.23456789 x 10 8
• For other calculators you much put it into
Scientific mode by pushing the “mode” button and selecting SCI then hitting enter.
• Get help with this after class or in tutoring.
2.8 Unit Equation and Unit Factor
Unit equation : 10 dimes = 1 dollar
Unit factor : 10 dimes
1 dollar or
1 dollar
10 dimes
Any equality (3 feet per 1 yard, 5 pennies per nickel, 12 inches per foot) can be written in fraction form for conversions.
Remember definitions don’t count as sig fig in calculations. Examples to follow.
2.9 Unit Analysis Problem Solving
1. What units need to be found?
2. Always START with the given
3. Multiply GIVEN by fractions so the units cancel until you get the final units
4. Round to the correct # of sig fig
SHOW ALL YOUR WORK IN
THIS CLASS FOR FULL CREDIT
(Note that exact numbers in conversions do not limit sig figs)
How many feet is 47.25 inches?
47.25 inches ( 1 foot / 12 inch) = 3.938 feet
Can someone explain?
Multiplying so focus on sig fig
Least sig fig is 4! The 1 foot / 12 inch is EXACT which means infinite sig fig (1.000000000… / 12.000000000…)
Definitions like 12 inches = 1 foot will not be “counted” in figuring out your sig fig. (12 inches is EXACTLY 1 foot)
Worksheets for each chapter
• http://web.gccaz.edu/~ksmith8/rev130.htm
• These types of problems are conversions, and we’ll do more in chapter 3. Start practicing NOW on them. Seriously.
• Also do all the practice problems in your online notes. Same web page.
• Your instructor may have even more
• See we expect you to already know how to do conversions, so will not spend much time in class on them, so YOU MUST practice a lot of problems at home!
2.10 The Percent Concept
Percent: Ratio of parts per 100 total parts.
(e.g. 80% is 80 parts/100 total parts )
To calculate Percent:
%
=
part whole
100 %
Given the percent, you can find the part or whole.
A chemistry class has 24 students. If 7 students are wearing red, what percent is wearing red?
29% or 29.2%
A coin collector has 5 silver dollars, 6 state quarters, and 3 Indian Head pennies. What percent is silver dollars?
36 or 35.7%
These problems ONLY have exact numbers, no measurements, so you can write how many sig figs you want. The next problem has measurements, so obey sig fig rules.
A crime lab finds 425 kilograms of meth that is 75.3% pure? How many kilograms of pure meth is there?
75.3% = (part pure / 425 kg total) x 100
Part pure = 3.20 x 10 2 kg (not 320, why?)
Need 3 sig fig