The Mole: A Measurement of Matter
advertisement

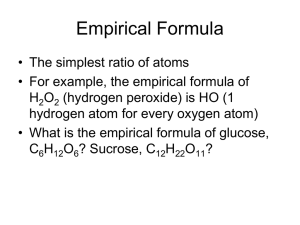
Chapter 7 The Mole: A Measurement of Matter At the end of this chapter, you should be able to: Describe how Avogadro’s number is related to a mole of any substance Calculate the mass of a mole of any substance The Mole (aka Avagadro’s Number): 6.02 x 23 10 The Mole and Avogadro’s Number SI unit that measures the amount of substance 1 mole = 6.02 x 1023 representative particles Representative particles are usually atoms, molecules, or formula units (ions) But Why the Mole? Just as 12 = 1 dozen, or 63,360 inches = 1 mile, the mole allows us to count microscopic items (atoms, ion, molecules) on a macroscopic scale. So, 1 mole of any substance is a set number of Items, namely: 6.02 x 1023. Chemistry = awesome Examples: Substance Representative Particle Chemical Formula Representative Particles in 1.00 mol Atomic nitrogen Atom N 6.02 x 1023 Water Molecule H2O 6.02 x 1023 Calcium ion Ion Ca2+ 6.02 x 1023 Solve Substance Nitrogen gas Calcium Fluoride Sucrose Carbon Representative Particle Chemical Formula Representative Particles in 1.00 mol Answers Nitrogen gas-molecule-N2 Calcium fluoride-formula unit-CaF2 Sucrose-molecule-C12H22O11 Carbon-atom-C All have 6.02 x 1023 representative particles in 1.00 mol How many atoms are in a mole? Determined from the chemical formula List the elements and count the atoms Solve for CO2 C - 1 carbon atom O - 2 oxygen atoms Add: 1 + 2 = 3 Answer: 3 times Avogadro’s number of atoms Solve: How many atoms are in a mole of 1. 2. 3. 4. Carbon monoxide – CO Glucose – C6H12O6 Propane – C3H8 Water – H2O How many moles of magnesium is 1.25 x 1023 atoms of magnesium? Refer to page 174 in text Divide the number of atoms or molecules given in the example by 6.02 x 1023 Divide (1.25 x 1023) by (6.02 x 1023) Express in scientific notation Answer = 2.08 x 10-1 mol Mg Objectives Use the molar mass to convert between mass and moles of a substance Use the mole to convert among measurements of mass, volume, and number of particles Molar mass Mass (in grams) of one mole of a substance Broad term (can be substituted) for gram atomic mass, gram formula mass, and gram molecular mass Can be unclear: What is the molar mass of oxygen? O or O2 ? - element O or molecular compound O2 ? Molar Mass Gram atomic mass (gam) – atomic mass of an element taken from the periodic table Gram molecular mass (gmm) – mass of one mole of a molecular compound Gram formula mass (gfm) – mass of one mole of an ionic compound Can use molar mass instead of gam, gmm, or gfm Calculating the Molar Mass of Compounds (Molecular and Ionic) 1. List the elements 2. Count the atoms 3. Multiply the number of atoms of the element by the atomic mass of the element (atomic mass is on the periodic table) 4. Add the masses of each element 5. Express to tenths place What is the molar mass (gfm) of ammonium carbonate (NH4)2CO3? N 2 x 14.0 g = 28.0 g H 8 x 1.0 g = 8.0 g C 1 x 12.0 g = 12.0 g O 3 x 16.0 g = 48.0 g Add ________ Answer 96.0 g Practice Problems 1. How many grams are in 9.45 mol of dinitrogen trioxide (N2O3) ? a. Calculate the grams in one mole b. Multiply the grams by the number of moles 2. Find the number of moles in 92.2 g of iron(III) oxide (Fe2O3). a. Calculate the grams in one mole b. Divide the given grams by the grams in one mole Answers 1. 718 g N2O3 (one mole is 76.0g) 2. 0.578 mol Fe2O3 (one mole is 159.6 g) Volume of a Mole of Gas Varies with a change in temperature or a change in pressure At STP, 1 mole of any gas occupies a volume of 22.4 L Standard temperature is 0°C Standard pressure is 101.3 kPa (kilopascals), or 1 atmosphere (atm) 22.4 L is known as the molar volume 22.4 L of any gas at STP contains 6.02 x 1023 representative particles of that gas One mole of a gaseous element and one mole of a gaseous compound both occupy a volume of 22.4 L at STP (Masses may differ) Study Figure 7.13 on page 186 Molar mass (g/mol) = Density (g/L) x Molar Volume (L/mol) Objectives Define the terms Calculate the percent composition of a substance from its chemical formula or experimental data Derive the empirical formula and the molecular formula of a compound from experimental data Terms to Know Percent composition – relative amounts of each element in a compound Empirical formula – lowest wholenumber ratio of the atoms of an element in a compound An 8.20 g piece of magnesium combines completely with 5.40 g of oxygen to form a compound. What is the percent composition of this compound? 1. Calculate the total mass 2. Divide each given by the total mass and then multiply by 100% 3. Check your answer: The percentages should total 100% Answer The total mass is 8.20 g + 5.40 g = 13.60 g Divide 8.2 g by 13.6 g and then multiply by 100% = 60.29412 = 60.3% Divide 5.4 g by 13.6 g and then multiply by 100% = 39.70588 = 39.7% Check your answer: 60.3% + 39.7% = 100% Calculate the percent composition of propane (C3H8) 1. List the elements 2. Count the atoms 3. Multiply the number of atoms of the element by the atomic mass of the element (atomic mass is on the periodic table) 4. Express each element as a percentage of the total molar mass 5. Check your answer Answer Total molar mass = 44.0 g/mol 36.0 g C = 81.8% 8.0 g H = 18.2% Calculate the mass of carbon in 82.0 g of propane (C3H8) 1. Calculate the percent composition using the formula (See previous problem) 2. Determine 81.8% of 82.0 g Move decimal two places to the left (.818 x 82 g) 3. Answer = 67.1 g Calculating Empirical Formulas Microscopic – atoms Macroscopic – moles of atoms Lowest whole-number ratio may not be the same as the compound formula Example: The empirical formula of hydrogen peroxide (H2O2) is HO Empirical Formulas The first step is to find the mole-to-mole ratio of the elements in the compound If the numbers are both whole numbers, these will be the subscripts of the elements in the formula If the whole numbers are identical, substitute the number 1 Example: C2H2 and C8H8 have an empirical formula of CH If either or both numbers are not whole numbers, numbers in the ratio must be multiplied by the same number to yield whole number subscripts What is the empirical formula of a compound that is 25.9% nitrogen and 74.1% oxygen? 1. Assume 100 g of the compound, so that there are 25.9 g N and 74.1 g O 2. Convert to mole-to-mole ratio: Divide each by mass of one mole 25.9 g divided by 14.0 g = 1.85 mol N 74.1 g divided by 16.0 g = 4.63 mol O 3. Divide both molar quantities by the smaller number of moles 4. 1.85/1.85 = 1 mol N 4.63/1.85 = 2.5 mol O 5. Multiply by a number that converts each to a whole number (In this case, the number is 2 because 2 x 2.5 = 5, which is the smallest whole number ) 2 x 1 mol N = 2 2 x 2.5 mol O = 5 Answer: The empirical formula is N 2O 5 Determine the Empirical Formulas 1. H2O2 2. CO2 3. N2H4 4. C6H12O6 5. What is the empirical formula of a compound that is 3.7% H, 44.4% C, and 51.9% N? Answers Compound 1. H2O2 2. CO2 3. N2H4 4. C6H12O6 5. HCN Empirical Formula HO CO2 NH2 CH2O Calculating Molecular Formulas The molar mass of a compound is a simple whole-number multiple of the molar mass of the empirical formula The molecular formula may or may not be the same as the empirical formula Calculate the molecular formula of the compound whose molar mass is 60.0 g and empirical formula is CH4N. 1. Using the empirical formula, calculate the empirical formula mass (efm) (Use the same procedure used to calculate molar mass.) 2. Divide the known molar mass by the efm 3. Multiply the formula subscripts by this value to get the molecular formula Answer Molar mass (efm) is 30.0 g 60.0 g divided by 30.0 g = 2 Answer: C2H8N2 The G.U.S. Method Allows students to organize data in an easy way. More importantly, makes my grading job easier. Works like so: G: the given. Write down all data given in the problem WITH proper units. U: the unknown. Write down what you are looking for AND the unit. S: solve. Show ALL work WITH units.