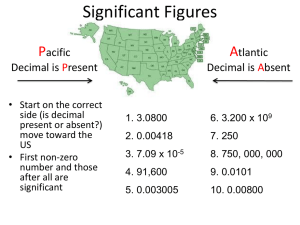
Chapter 2
advertisement

Chapter 2 Analyzing Data Chapter 2 Introduction • 2.1 Units & Measurements • 2.2 Scientific Notation & Dimensional Analysis – “Factor-Label” Method a.k.a. Conversion Factors or Dimensional Analysis • 2.3 Uncertainty in Data – Sig Figs, Sig Digs Chapter 2 Learning Targets By the end of Chapter 2 I am able to… Identify the SI base units of measurements for mass, time, length, temperature & volume (2.1) Distinguish between qualitative & quantitative observations and give examples of each. (2.1) Explain the meanings of the SI prefixes (2.1) Compare & contrast mass and weight (2.1) State the derived units used to represent speed, area & density. (2.1) Analyze a problem, solve for an unknown and evaluate my answer (2.1) Chapter 2 Learning Targets Express numbers in scientific notation (2.2) Convert between units using dimensional analysis/factor-label method (2.2) Define & compare accuracy & precision (2.3) Describe the accuracy of experimental data using error & percent error (2.3) Apply the rules of “Sig Figs” to express uncertainty in measured & calculated values (2.3) 2.1 Units & Measurements Learning Targets Identify the SI base units of measurements for mass, time, length, temperature & volume (2.1) Distinguish between qualitative & quantitative observations and give examples of each. (2.1) Explain the meanings of the SI prefixes (2.1) Compare & contrast mass and weight (2.1) State the derived units used to represent speed, area & density. (2.1) Analyze a problem, solve for an unknown and evaluate my answer (2.1) 2.1 Units & Measurements • Système Internationale d'Unités (SI) is an internationally agreed upon system of measurements. • Chemistry involves both measuring and calculating • Two types of observations in science – Qualitative (no measurements, no numbers) – Quantitative (actual measurements) 2.1 Units & Measurements • There are 7 base units in SI • Measurements based on an object or event (a physical standard). • See p. 33 in text. • YOU NEED TO KNOW THESE! 2.1 Units & Measurements • To better describe the range of possible measurements, scientists add prefixes to base units • Based on factors of 10 – metric system ** KNOW THESE… p 33 in text 2.1 Units & Measurements • Mass vs. Weight – Weight is a measure of force of gravity between two objects (wt. changes w/respect to gravity) • Scales are for weighing – Mass is a measure of the amount of matter an object contains • A balance is used for finding mass • The SI unit for mass is the Kilogram (kg) The Physical Standard for Mass (FYI…) • The international prototype of the kilogram is inside three nested bell jars at the Bureau International des Poids et Mesures in Paris. http://www.npr.org/templates/story/story.php?storyId=112003322 In search of a new Standard… • Physicist Richard Steiner adjusts the watt balance. This extremely sensitive scale can detect changes as small as ten-billionths of a kilogram. 2.1 Units & Measurements • Temperature: quantitative measurement of the average kinetic energy of the particles w/in an object. • A thermometer is used to measure temperature • Three temperature scales: – Fahrenheit, Celsius, Kelvin • SI base unit - Kelvin 2.1 Units & Measurements • Kelvin scale developed by William Thomson (a.k.a. Lord Kelvin) • Zero Kelvin is the point at which all molecular motion stops – “Absolute Zero” • The size of the Celsius degree (oC) is the same as a Kelvin (K) • To convert between the two: K C -273 C K +273 Water boils at 100 oC, to convert to Kelvin add 273. What is water’s BP in K? 373 K 2.1 Units & Measurements • Derived Units: combination of base units • Volume – SI unit is cubic meter (m3) – Usually the liter (L) is used – 1 L equals 1 dm3 – For laboratory use the cubic centimeter is often used (cm3 or cc) – 1 cm3 = 1 mL (See Figure 2.4 p. 36) Figure 2.4 p. 36 The three cubes show volume relationships between m3 dm3 & cm3. as you move from left to right, the volume of each cube gets 10 x 10 x 10, or 1000 (103) times smaller. 2.1 Units & Measurements • Derived Units… continued • Density – a physical property of matter – defined as amount of mass per unit volume (density = mass/volume) Common units: – g/cm3 for solids – g/ml for liquids & gases Application Question: 116 g of sunflower oil is used in a recipe. The density of the oil is 0.925 g/ml. What is the volume of the sunflower oil in ml? What are you being asked to solve for? volume of sunflower oil What do you know, what are you given? Density = mass/volume Density = 0.925 g/ml Mass = 116 g What is the unknown? volume Write the equation and isolate the unknown factor. density = mass/volume rearrange to solve for unknown: volume = mass/density Substitute known quantities into equation & solve. volume = 116g/0.925g/ml volume = 125 ml 2.1 HW problems • #2-6 (p.38-39) , #66-67 (p. 62) • Due : – Next class 2.2 Scientific Notation & Dimensional Analysis Learning Targets Express numbers in scientific notation (2.2) Convert between units using dimensional analysis/factor-label method (2.2) 2.2 Scientific Notation & Dimensional Analysis • Scientific notation used for short-handing very large and very small measurements. • Very large number - the number of atoms in a sample might be something like 124,500,000,000,000 atoms. • Very small number - the size of an molecule in meters might be something like 0.0000000000238 meters. 2.2 Scientific Notation & Dimensional Analysis • The number of places moved equals the value of the exponent. • The exponent is positive when the decimal moves to the left and negative when the decimal moves to the right. • Example: Exponent 800 = 8.0 102 0.0000343 = 3.43 10–5 Coefficient 2.2 Application • Question: Each cell in the human body contains a complete genome which is composed of base pairs. Each base pair is 0.000,000,034m in length. There are 6,000,000,000 base pairs in each human cell. Change the above information into scientific notation. a.) 3.4 x 10-8 m b.) 6 x 109 base pairs 2.2 Scientific Notation & Dimensional Analysis • Addition & Subtraction of numbers in scientific notation: – Exponents must be the same. – Add or subtract coefficients. (7.35 x 102 m) + (2.43 x 102 m) = 9.78 x 102 m Application • Add 3.5 x 103 m to 6.8 x 103 m NO, you can’t write it like this! (3.5 x 103) + (6.8 x 103) = 10.3 x 103m Why??? Answer must be 1.03 x 104 because proper scientific notation states that you must have one whole number to the left of the decimal 2.2 Scientific Notation & Dimensional Analysis • What if the exponents are NOT the same? – Rewrite values with the same exponent. – Example: Consider amounts of energy produced by renewable energy sources in the U.S. in 2004: • • • • • Hydroelectric Biomass Geothermal Wind Solar What is the Total ? 2.840 x 1018 J 3.146 x 1018 J 3.60 x1017 J 1.50 x 1017 J 6.9 x 1016 J 0.360 x 1018 J 0.150 x 1018 J 0.069 x 1018 J 6.565 x 1018 J Application • Subtract 7.9 x 102 km from 1.0 x 103 km Move decimal place to the Left to make exponents the same, 103 NO, you can’t write it like this! (1.0 x 103) – (0.79 x 103) = 0.21 x 103 Remember you must write as 2.1 x 102 (one whole number to the left of the decimal!!) 2.2 Scientific Notation & Dimensional Analysis • Multiplication and division, exponents do NOT need to be the same: – To multiply, multiply the coefficients, then add the exponents. (4.6 x 1023 atoms) (2x10-23 g/atom) = 9.2 x 100 g = 9.2 g – To divide, divide the coefficients, then subtract the exponent of the divisor from the exponent of the dividend. (9 x 108) / (3 x 10-4) Divide coefficients: 9/3 = 3 Subtract the exponents: 8 – (-4) = 8+4 = 12 Combine the parts: 3 x 1012 Math Skill Review • 1. 2. 3. 4. 5. Can you multiply these fractions? Complete the following in your notebook. Remember… MATH IN PENCIL! 2/3 x 5/7 2/3 x 3/9 a/b x c/d a2/b x b3/a 5 x 2/15 2.2 Scientific Notation & Dimensional Analysis • • “Factor-Label Method” (Dimensional Analysis & Conversion factors – book name) Problem solving consists of three parts: Known Conversion Factor Desired Answer 2.2 Scientific Notation & Dimensional Analysis • Conversion factors are ratios with a value equal to one • Example: $1 = 4 quarters 1km = 1000m • The ratios are written as follows: $1 and 4 quarters 4 quarters $1 1 km 1000 m and 1000 m 1 km 2.2 Application • An object is traveling at a speed of 7500 centimeters per second. Convert the value to kilometers per minute. Known: 7500 cm /sec Desired: ? km/min • What relationships are known between cm & km? Between sec & min? Write them down 100 cm = 1m; 1000 m = 1 km; 60 s = 1 min • Use these relationships as ratios in such a way that s, cm, & m all divide out: • km = min Open Note Quiz 1. How many seconds in a class at SKHS? Class periods are 98 minutes. 2. Convert 78 seconds to hours 3. Convert 2.5 x 106 g to kg 4. Convert 37.5g/ml to kg/L 5. Convert 7.56 mm3/s to dm3/min 6. Convert 9.06 km/hr to m/s 2.2 HW – Due Next Class • • • • #11-16 (p. 41-43) #19-20 (p. 45) #25 (p. 46) #76-80 (p. 62) 2.3 Uncertainty in Data Learning Targets Define & compare accuracy & precision (2.3) Describe the accuracy of experimental data using error & percent error (2.3) Apply the rules of “Sig Figs” to express uncertainty in measured & calculated values (2.3) 2.3 Uncertainty in Data • Accuracy & Precision – Accuracy refers to how close a measured value is to an accepted value – Precision refers to how close measurements are to one another. 2.3 Uncertainty in Data Figure 2.10 on p. 47 2.3 Uncertainty in Data Application • Open your books and consider the data table, p. 48. 2.3 Uncertainty in Data Application - continued • Students were asked to determine the density of an unknown white powder. • Each student measured the volume and mass of three samples. • They calculated the densities and averaged the three. 2.3 Uncertainty in Data Application - continued • Which student collected the most accurate data? • Student A • Why? – closest to the accepted value. • • • • Who collected the most precise data? Student C Why? closest to one another. 2.3 Uncertainty in Data • Error & Percent Error – Error is defined as the difference between an experimental value (values measured during an experiment) and an accepted value – Error Equation: Error = experimental value – accepted value Absolute value… only concerned about how far away from the target you were, so sign doesn’t matter 2.3 Uncertainty in Data • Error & Percent Error (cont…) – Percent Error expresses error as a percentage of the accepted value. – Percent Error Equation: Absolute value is used because only the size of the error matters; it does not matter whether the experimental value is larger or smaller than the accepted value. 2.3 Application – do this in your notebook as part of your notes • The melting point of paradichlorobenzene is 53oC. In a laboratory activity two students tried to verify this value. Student #1 records: 51.5oC, 53.5oC, 55.0oC, 52.3oC, and 54.2oC Student #2 records: 52.3oC, 53.2oC, 54.0oC, 52.5oC, and 53.5oC a. Calculate the average value for the two students b. Calculate the percent error for each student c. Which of the students is most precise? Accurate? Explain. Calculate the average value for the two students Student #1: 51.5oC + 53.5oC + 55.0oC + 52.3oC + 54.2oC = 266.5/5 = 53.3oC Student #2 : 52.3oC + 53.2oC + 54.0oC + 52.5oC + 53.5oC = 53.1oC Calculate the percent error for each student • Student #1: Percent error = • Student #2: Percent error = (53.3oC - 53.0oC) X100 = 0.566 % error 53.0oC (53.1oC - 53.0oC) X100 = 0.189 % error 53.0oC Which of the students is most precise? Accurate? Explain. • Student 2 is the most precise with a range of values from 52.3 to 54.0. • Student 2 is also most accurate with a 0.189 % error. 2.3 Uncertainty in Data • Significant Figures: include all known digits plus one estimated digit. • Often precision is limited by the tools available. 5.00 cm Figure 2.12 p. 50 The markings on the ruler represent the known digits plus the estimated digit. The measure is 5.23 cm. What is the estimated digit if the length of the object fell directly on the 5 cm mark? 2.3 Uncertainty in Data • Rules for Significant Figures: –Rule 1: Nonzero numbers are always significant. •Example: 72.3 g How many sig figs? 3 • 9.4567 How many sig figs? 5 –Rule 2: Zeros between nonzero numbers are always significant. •Example: 60.5 g How many sig figs? 3 • 5005.05 How many sig figs? 6 –Rule 3: All final zeros to the right of the decimal are significant. •Example: 6.2000 How many sig figs? 5 2.3 Uncertainty in Data • Rules for Significant Figures: – Rule 4: Placeholder zeros are not significant. To remove placeholder zeros, rewrite the number in scientific notation. • Example: 0.0253g and 4320 (3 sig figs each) Rewritten in Scientific notation: 2.53 x 10-2 4.32 x 103 0.000601 How many sig figs? 3 6.01 x 10-4 50000 How many sig figs? 1 5 x 104 2.3 Uncertainty in Data • Rules for Significant Figures: –Rule #5: Counting numbers and defined constants have an infinite number of significant figures. –Example: 6 molecules 60s = 1 min 2.3 Uncertainty in Data Examples using the 4 Rules: 5465 0.60750 0.020020 500.0 300 How many Sig Figs 4 – Rule #1 5 – Rules #2 & 3 5 – Rules #2 & 3 4 – Rules #2 & 3 1 – Rule #4 2.3 Uncertainty in Data • Rules for Significant Figures: – What happens when your calculator gives you a funky number, how do you know how many sig figs to report in your answer? – Rule 6: Addition & Subtraction, answer will have the number of sig figs from the number with the least amount of decimal places in the problem. Example: Decimal Places 10.21 2 0.2 1 + 256 266.41 So… my answer should have 0 decimal places 0 266 2.3 Uncertainty in Data • Rules for Significant Figures: – Rule 7: Multiplication & Division The answer will have the number of sig figs from the number with the least amount of sig figs. Example: **YOU WILL USE THIS RULE 4675 x 625 = ________ A LOT!!!!! Which has the least # of sig figs? 625 has 3, so your answer must have 3 4675 x 625 = 2921875 2920000 = 2.92 x 106 3 sig figs Rule 4 2.3 Homework • • • • #32-36 p. 49-51 #40 p. 53 # 42-43, 47 & 51 p. 54 #87, 91, 93-94 p. 63