Matter is classified into three states. Which answer contains all of the
advertisement
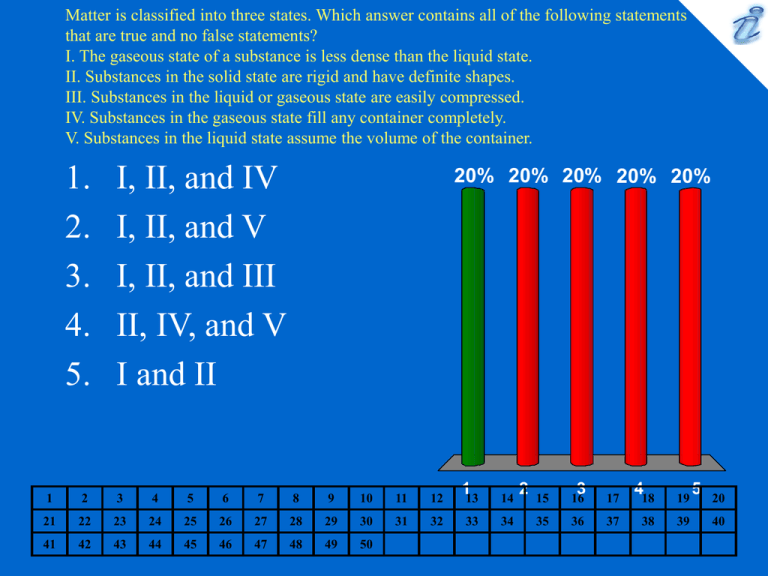
Matter is classified into three states. Which answer contains all of the following statements that are true and no false statements? I. The gaseous state of a substance is less dense than the liquid state. II. Substances in the solid state are rigid and have definite shapes. III. Substances in the liquid or gaseous state are easily compressed. IV. Substances in the gaseous state fill any container completely. V. Substances in the liquid state assume the volume of the container. 1. 2. 3. 4. 5. I, II, and IV I, II, and V I, II, and III II, IV, and V I and II 20% 20% 20% 20% 20% 1 2 3 4 5 6 7 8 9 10 11 12 113 14 21 22 23 24 25 26 27 28 29 30 31 32 33 34 41 42 43 44 45 46 47 48 49 50 2 15 16 3 17 418 19 35 36 37 38 39 5 20 40 Which answer includes all the following that are chemical changes and no physical changes? I. freezing of water II. rusting of iron III. dropping a piece of iron into hydrochloric acid (H2 is produced) IV. burning a piece of wood V. emission of light by a kerosene oil lamp 1. 2. 3. 4. 5. III and IV II and V I, II, III, IV, and V II, III, and V II, III, IV, and V 20% 20% 20% 20% 20% 1 2 3 4 5 6 7 8 9 10 11 12 113 14 21 22 23 24 25 26 27 28 29 30 31 32 33 34 41 42 43 44 45 46 47 48 49 50 2 15 16 3 17 418 19 35 36 37 38 39 5 20 40 Choose the response that includes all things listed below that are pure substances and no others. I. Steel II. sugar water III. Oxygen IV. Steam V. sodium nitrite VI. gunpowder 1. 2. 3. 4. 5. II, III, and VI I and III III and V III, IV, and V II and VI 20% 20% 20% 20% 20% 1 2 3 4 5 6 7 8 9 10 11 12 113 14 21 22 23 24 25 26 27 28 29 30 31 32 33 34 41 42 43 44 45 46 47 48 49 50 2 15 16 3 17 418 19 35 36 37 38 39 5 20 40 Trying to identify a sample of a pure substance, a student makes the following observations: I. It has a mass of 5400 g. II. It is 10. cm long, 10. cm wide, and its height is 20. cm. III. It is a shiny solid at room temperature. IV. It dissolves in hydrochloric acid. V. It melts at 660°C. VI. It is a good conductor of electricity. Which response includes all of these observations that, individually or in combination, would be helpful in identifying the substance of which the sample is composed? 1. 2. 3. 4. 5. 20% 20% 20% 20% 20% I, III, IV, and V II, IV, V, and VI III and IV III, IV, and VI I, II, III, IV, V, and VI 1 2 3 4 5 6 7 8 9 10 11 12 113 14 21 22 23 24 25 26 27 28 29 30 31 32 33 34 41 42 43 44 45 46 47 48 49 50 2 15 16 3 17 418 19 35 36 37 38 39 5 20 40 What is the symbol for the element copper? 1. 2. 3. 4. 5. C Co Cm Cu Cr 20% 20% 20% 20% 20% 1 2 3 4 5 6 7 8 9 10 11 12 113 14 21 22 23 24 25 26 27 28 29 30 31 32 33 34 41 42 43 44 45 46 47 48 49 50 2 15 16 3 17 418 19 35 36 37 38 39 5 20 40 Which name - symbol combination is wrong? 1. 2. 3. 4. 5. silver - Au krypton - Kr zinc - Zn platinum - Pt tungsten - W 20% 20% 20% 20% 20% 1 2 3 4 5 6 7 8 9 10 11 12 113 14 21 22 23 24 25 26 27 28 29 30 31 32 33 34 41 42 43 44 45 46 47 48 49 50 2 15 16 3 17 418 19 35 36 37 38 39 5 20 40 Which one of the following lists units of volume in increasing order, i.e., from smallest to largest? 1. microliter < kiloliter < centiliter < liter < mililiter milliliter < centiliter < microliter < kiloliter < liter centiliter < mililiter < liter < kiloliter < microliter microliter < mililiter < centiliter < liter < kiloliter milliliter < centiliter < liter < kiloliter < microliter 2. 3. 4. 5. 20% 20% 20% 20% 20% 1 2 3 4 5 6 7 8 9 10 11 12 113 14 21 22 23 24 25 26 27 28 29 30 31 32 33 34 41 42 43 44 45 46 47 48 49 50 2 15 16 3 17 418 19 35 36 37 38 39 5 20 40 Which one of the following is closest to one centimeter? 1. the diameter of an apple 2. the width of a standard door 3. the diameter of a dime 4. the length of a football field 5. the diameter of a typical apple pie 20% 20% 20% 20% 20% 1 2 3 4 5 6 7 8 9 10 11 12 113 14 21 22 23 24 25 26 27 28 29 30 31 32 33 34 41 42 43 44 45 46 47 48 49 50 2 15 16 3 17 418 19 35 36 37 38 39 5 20 40 Perform the indicated mathematical operations and round off the answer to the proper number of significant figures: (12.67 x 4.23) ÷ 23.42 = 1. 2. 3. 4. 5. 2.3 2.29 2.288 2.88 2.2884 20% 20% 20% 20% 20% 1 2 3 4 5 6 7 8 9 10 11 12 113 14 21 22 23 24 25 26 27 28 29 30 31 32 33 34 41 42 43 44 45 46 47 48 49 50 2 15 16 3 17 418 19 35 36 37 38 39 5 20 40 How many milliliters are there in 1.0 microliter? 1. 2. 3. 4. 5. 1.0 x 10-1 mL 1.0 x 10-2 mL 1.0 x 10-3 mL 1.0 x 10-4 mL 1.0 x 10-5 mL 20% 20% 20% 20% 20% 1 2 3 4 5 6 7 8 9 10 11 12 113 14 21 22 23 24 25 26 27 28 29 30 31 32 33 34 41 42 43 44 45 46 47 48 49 50 2 15 16 3 17 418 19 35 36 37 38 39 5 20 40 What is the area (in mm2) of a rectangle that is 6.04 cm wide and 8.16 inches long? 1. 2. 3. 4. 5. 19.4 mm2 1.25 x 104 mm2 1.94 x 103 mm2 1.25 x 103 mm2 125 mm2 20% 20% 20% 20% 20% 1 2 3 4 5 6 7 8 9 10 11 12 113 14 21 22 23 24 25 26 27 28 29 30 31 32 33 34 41 42 43 44 45 46 47 48 49 50 2 15 16 3 17 418 19 35 36 37 38 39 5 20 40 1. 2. 3. 4. 5. 5.24 x 10-22in3 1.95 x 10-24in3 3.25 x 10-8in3 68.6 in3 8.16 x 10-12 in3 20% 20% 20% 20% 20% 1 2 3 4 5 6 7 8 9 10 11 12 113 14 21 22 23 24 25 26 27 28 29 30 31 32 33 34 41 42 43 44 45 46 47 48 49 50 2 15 16 3 17 418 19 35 36 37 38 39 5 20 40 Stainless 316 is a steel alloy containing 17% Cr, 12% Ni, 3.0% Mo, and 0.10% C with the rest being Fe. What mass of iron (in kg) would be contained in 1.00 metric tonne of this alloy? 1 metric tonne = 103 kg. 1. 2. 3. 4. 5. 68 kg 760 kg 580 kg 680 kg 780 kg 20% 20% 20% 20% 20% 1 2 3 4 5 6 7 8 9 10 11 12 113 14 21 22 23 24 25 26 27 28 29 30 31 32 33 34 41 42 43 44 45 46 47 48 49 50 2 15 16 3 17 418 19 35 36 37 38 39 5 20 40 What is the mass of 35.0 mL of a liquid with a specific gravity of 2.64? 1. 2. 3. 4. 5. 35.0 g 13.3 g 26.2 g 92.4 g 0.0754 g 20% 20% 20% 20% 20% 1 2 3 4 5 6 7 8 9 10 11 12 113 14 21 22 23 24 25 26 27 28 29 30 31 32 33 34 41 42 43 44 45 46 47 48 49 50 2 15 16 3 17 418 19 35 36 37 38 39 5 20 40 The normal boiling point of zinc is 788°F. What is this in °C ? 1. 2. 3. 4. 5. 251°C 1450°C 724°C 962°C 420°C 20% 20% 20% 20% 20% 1 2 3 4 5 6 7 8 9 10 11 12 113 14 21 22 23 24 25 26 27 28 29 30 31 32 33 34 41 42 43 44 45 46 47 48 49 50 2 15 16 3 17 418 19 35 36 37 38 39 5 20 40 What is the final temperature of the chromium and water combination when 50.0 g of chromium at 15°C (specific heat = 0.448 J/g•°C) is added to 25 mL of water (specific heat = 4.18 J/g•°C) at 45°C? 1. 2. 3. 4. 5. 20°C 25°C 30°C 35°C 40°C 20% 20% 20% 20% 20% 1 2 3 4 5 6 7 8 9 10 11 12 113 14 21 22 23 24 25 26 27 28 29 30 31 32 33 34 41 42 43 44 45 46 47 48 49 50 2 15 16 3 17 418 19 35 36 37 38 39 5 20 40