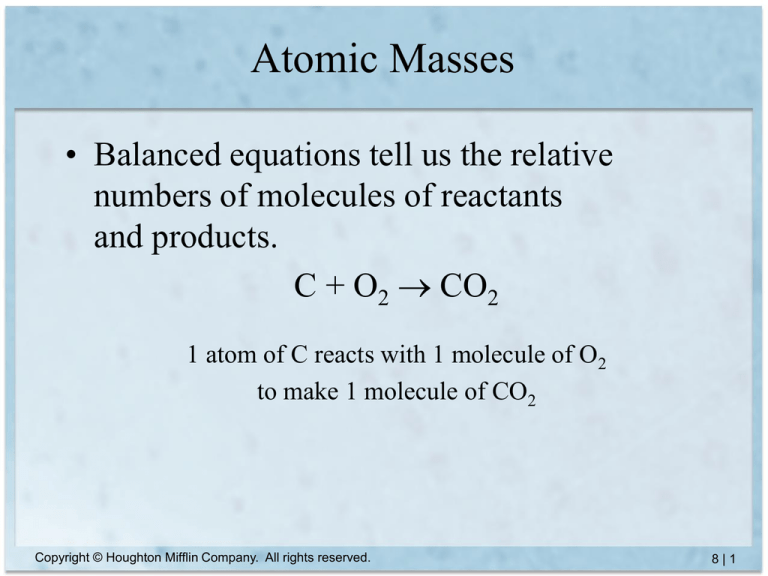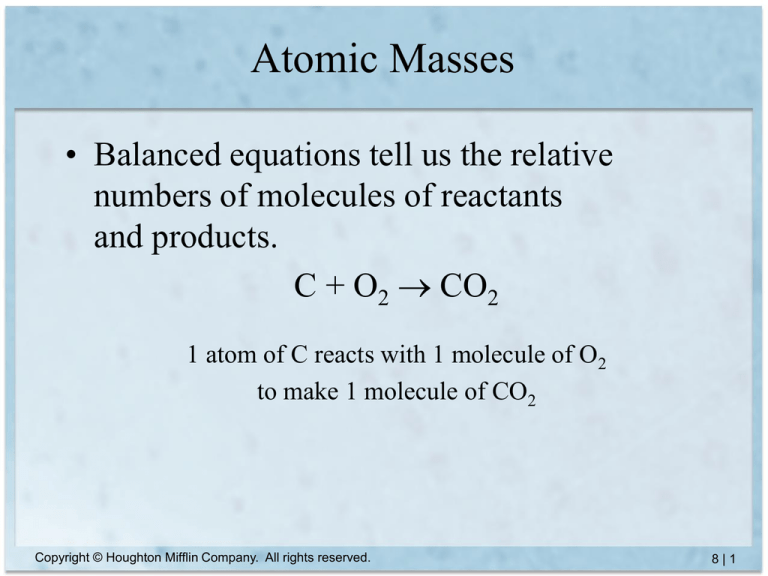
Atomic Masses
• Balanced equations tell us the relative
numbers of molecules of reactants
and products.
C + O2 CO2
1 atom of C reacts with 1 molecule of O2
to make 1 molecule of CO2
Copyright © Houghton Mifflin Company. All rights reserved.
8|1
Atomic Masses (cont.)
• If you want to know how many O2 molecules you will need,
or how many CO2 molecules you can make, you will need to
know how many C atoms are in the sample.
• You can either count the atoms (not possible) or weigh the
carbon and calculate the number of atoms. You can do this if
you know the mass of one atom of carbon.
• Atomic masses allow us to convert weights into numbers of
atoms.
• The unit is the amu (atomic mass unit)
1 amu = 1.66 x 10-24g
Copyright © Houghton Mifflin Company. All rights reserved.
8|2
Atomic Masses (cont.)
Copyright © Houghton Mifflin Company. All rights reserved.
8|3
Atomic Masses (cont.)
• If our sample of carbon weighs 3.00 x 1020 amu,
how many carbon atoms are present?
Since our equation tells us that 1 C atom reacts
with 1 O2 molecule, if we have 2.50 x 1019 C
atoms, how many molecules of O2 will we need?
Copyright © Houghton Mifflin Company. All rights reserved.
8|4
Moles
• The mass of 1.0 mole of an element is equal to its atomic
mass in grams.
• One mole = 6.022 x 1023 units. This number is called
Avogadro’s number.
• One mole of carbon-12 atoms weighs 12.0 g and has
6.02 x 1023 atoms.
• One atom of carbon-12 weighs 12.0 amu.
Copyright © Houghton Mifflin Company. All rights reserved.
8|5
Compute the number of moles and the number of
atoms in 10.0 g of Al.
Use the periodic table to determine the mass of 1 mole
of Al.
•
Use this as a conversion factor for converting
grams to moles.
•
Copyright © Houghton Mifflin Company. All rights reserved.
8|6
• Use Avogadro’s Number to determine the number of atoms in
1 mole.
• Use this as a conversion factor for converting moles to atoms.
Copyright © Houghton Mifflin Company. All rights reserved.
8|7
Compute the number of moles and the mass
in g of 2.23 x 1023 atoms of Al.
• Use Avogadro’s Number to determine the number of atoms
in 1 mole.
• Use this as a conversion factor for converting atoms to
moles.
Copyright © Houghton Mifflin Company. All rights reserved.
8|8
• Use the periodic table to determine the mass of 1 mole of Al.
• Use this as a conversion factor for converting moles to
grams.
Copyright © Houghton Mifflin Company. All rights reserved.
8|9
Molar Mass
• Molar mass: the mass in grams of one mole of a
compound
• The relative weights of molecules can be
calculated from atomic masses.
water = H2O = 2(1.008 amu) + 16.00 amu =
18.02 amu
• 1 mole of H2O will weigh 18.02 g, therefore the
molar mass of H2O is 18.02 g.
• 1 mole of H2O will contain 16.00 g of oxygen
and 2.02 g of hydrogen.
Copyright © Houghton Mifflin Company. All rights reserved.
8 | 10
A 12 oz Pepsi contains approximately 20 g of sucrose, whose
molecular formula is C12H22O11. How many molecules of
sucrose are present?
Copyright © Houghton Mifflin Company. All rights reserved.
8 | 11
Percent Composition
• Percentage by mass of each element in a compound
• Can be determined from the formula of the
compound.
• The percentages may not always total to 100% due
to rounding.
part
Percentage
100%
whole
Copyright © Houghton Mifflin Company. All rights reserved.
8 | 12
Determine the percent composition of each element in
the compound C2H5OH.
•
Determine the mass of each element in 1 mole of the
compound.
•
Determine the molar mass of the compound by adding the
masses of the elements.
Copyright © Houghton Mifflin Company. All rights reserved.
8 | 13
• Divide the mass of each element by the molar mass
of the compound and multiply by 100%
Copyright © Houghton Mifflin Company. All rights reserved.
8 | 14
Calculate the mass % of each element in Ca(NO3)2.
Copyright © Houghton Mifflin Company. All rights reserved.
8 | 15
Calculate the mass of N in grams present in 5g of
glycine (C2H5O2N)
Copyright © Houghton Mifflin Company. All rights reserved.
8 | 16
Empirical Formulas
• Empirical formula: the simplest, whole-number ratio of
atoms in a molecule
• Molecular formula: a multiple of the empirical formula
• Examples:
Compound
Empirical Formula Molecular Formula
Benzene
Hydrogen peroxide
Water
CH
HO
H2O
Copyright © Houghton Mifflin Company. All rights reserved.
C6H6
H2O2
H2O
8 | 17
Determine the empirical formula of benzopyrene, C20H12
Find the greatest common factor (GCF) of the subscripts.
Divide each subscript by the GCF to get the empirical formula.
Empirical Formula =
Copyright © Houghton Mifflin Company. All rights reserved.
8 | 18
Determining the empirical formula from grams
• Obtain the mass of each element in g.
• Determine the number of moles of each element.
• Divide the number of moles of each element by the smallest
number of moles present.
• If a non-integer results, multiply all the numbers by the
smallest number integer that converts all of the numbers into
whole number integers.
Copyright © Houghton Mifflin Company. All rights reserved.
8 | 19
When 3.269 g of Zn is heated in pure oxygen, the sample gains
0.8 g of oxygen in forming the corresponding oxide. Calculate its
empirical formula.
Copyright © Houghton Mifflin Company. All rights reserved.
8 | 20
When 1 g of chromium metal is heated with chlorine gas, 3.045g of a
chromium chloride salt results. Calculate the empirical formula of the salt.
Copyright © Houghton Mifflin Company. All rights reserved.
8 | 21
Determine the empirical formula of acetic anhydride if its percent
composition is 47% carbon, 6.0% hydrogen, and 47% oxygen.
• Convert the percentages to grams by assuming you have 100 g of the compound.
•
Convert the grams to moles.
•
Divide each by the smallest number of moles.
Copyright © Houghton Mifflin Company. All rights reserved.
8 | 22
• If any of the ratios is not a whole number, multiply all the ratios by a factor
to make it a whole number.
• Use the ratios as the subscripts in the empirical formula.
Copyright © Houghton Mifflin Company. All rights reserved.
8 | 23
Calculate the empirical formula of a compound that contains
40% carbon, 6.7% hydrogen, and 53.3% oxygen.
Copyright © Houghton Mifflin Company. All rights reserved.
8 | 24
Molecular Formulas
• The molecular formula is a multiple of the
empirical formula.
• To determine the molecular formula you
must know the empirical formula and the
molar mass of the compound.
Copyright © Houghton Mifflin Company. All rights reserved.
8 | 25
Determine the molecular formula of benzopyrene if it has a molar
mass of 252 g and an empirical formula of C5H3
Determine the molar mass of the empirical formula.
Divide the given molar mass of the compound by the molar mass of the empirical formula
and round to the nearest whole number.
Multiply the empirical formula by the calculated factor to give the molecular formula.
Copyright © Houghton Mifflin Company. All rights reserved.
8 | 26
If a compound with an empirical formula CH2O has a
molar mass of 180.16, what is its molecular formula?
Copyright © Houghton Mifflin Company. All rights reserved.
8 | 27