What are physical properties of matter?
advertisement
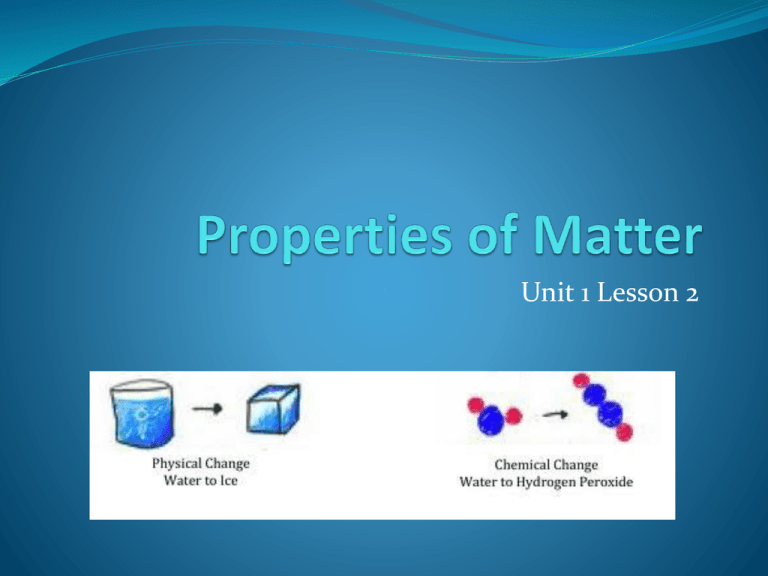
Unit 1 Lesson 2 What are the Properties of Matter? Matter can have many different properties, or characteristics. Materials can be hard or soft, rough or smooth, hot or cold, liquid, solid, or gas. Chemistry is the study of the properties of matter and how matter changes. What is a Substance? The properties of matter depend on its makeup. Some types of matter are substances and some are not. A substance is a single kind of matter that is pure – it always has a specific composition and a specific set of properties. Example is salt – no matter where it comes from – seawater or a salt mine – it always has the same composition and properties. What are the two properties of matter? Every form of matter has 2 kinds of properties – physical and chemical. For example: A physical property of oxygen (O2) is that it is a gas at room temperature. A chemical property of oxygen (O2) is that it reacts with iron to form rust. What are physical properties of matter? The physical properties of matter help to identify and classify matter in its different forms. A physical property is a characteristic of a pure substance that can be observed without changing it into another substance. All of the senses can be used to observe physical properties. For example: A physical property of water is that it freezes at a temperature of O0C. When liquid water freezes it changes to solid ice, but it is still water. Unit 1 Lesson 2 Properties of Matter What are physical properties of matter? • Mass and volume are physical properties. • Changing the mass or volume of a substance does not change the substance’s identity. • The state of matter is a physical property. The state of matter is the physical form of the matter. • Most matter exists as a solid, liquid, gas, or plasma. Copyright © Houghton Mifflin Harcourt Publishing Company Unit 1 Lesson 2 Properties of Matter What are physical properties of matter? • Electrical conductivity is a measure of how well electric currents move through a substance. • Density is the measure of the amount of matter in a given volume. • Thermal conductivity is the rate at which a substance transfers heat. Copyright © Houghton Mifflin Harcourt Publishing Company What are Physical Properties of Matter? Solubility is the ability of a substance to dissolve in another substance. Whether or not a substance dissolves in water is also a physical property. For example, sugar will dissolve in water, but iron will not. What are Physical Properties of matter? Malleability is the ability of a substance to be rolled or pounded into various shapes. Magnetic attraction is also a physical property that can be observed. Some metals, such as iron, are magnetized – they can be attracted to a magnet. Unit 1 Lesson 2 Properties of Matter What are physical properties of matter? • The shine, or luster, of a metal can be easily observed. • The melting point of a substance is the temperature at which it changes from a solid to a liquid. • The boiling point of a substance is the point at which the substance boils (liquid to gas). Copyright © Houghton Mifflin Harcourt Publishing Company What are Chemical Properties of Matter? Unlike physical properties of matter, some properties can’t be observed just by looking at or touching a substance. • A chemical property describes the ability of a substance to change into a new substance with different properties. • The ability to rust or tarnish is a chemical property. When a metal rusts or tarnishes, it changes to a different substance. What are Chemical Properties of Matter? One chemical property of iron is that it will combine slowly with oxygen in the air to from a different substance – rust. Silver will react with sulfur in the air to form tarnish. In contrast a chemical property of gold is that it does not react easily with oxygen or sulfur. Unit 1 Lesson 2 Properties of Matter What are chemical properties of matter? • Chemical properties can be identified by the changes they produce. • Flammability is the ability of a substance to burn. • Reactivity is the ability of a substance to interact with another substance and form one or more new substances. Copyright © Houghton Mifflin Harcourt Publishing Company Unit 1 Lesson 2 Properties of Matter Property Boundaries What is the difference between physical and chemical properties? • Physical properties can be observed without changing the identity of a substance. • Chemical properties can be observed only by changing the identity of a substance. Copyright © Houghton Mifflin Harcourt Publishing Company Unit 1 Lesson 2 Properties of Matter At the Scene • The collection and study of physical evidence in a criminal investigation is known as forensic science. • Ashes from an arson scene can be heated to determine chemicals used to start a fire. • Flecks of paint and the analysis of fibers can provide clues to criminal cases. Copyright © Houghton Mifflin Harcourt Publishing Company Unit 1 Lesson 2 Properties of Matter Identify Yourself How can physical and chemical properties identify a substance? • Properties unique to a substance are its characteristic properties. • Characteristic properties stay the same regardless of the amount of the sample. They can help identify a substance. • Characteristic properties can be physical properties such as density, or chemical properties, such as flammability. Copyright © Houghton Mifflin Harcourt Publishing Company