Transition point - St John Brebeuf
advertisement

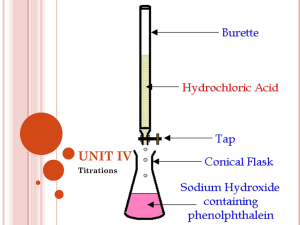
Acids/Bases Lesson 11 Indicators Theory Ishihara Test for Colour Blindness – if you can read all of the numbers you have good colour vision Indicators Indicators are weak organic acids or bases that display different colours for their conjugate acid and base forms.. They are such complex molecules that their names are usually abbreviated HIn : an acid and an indicator In- : a base and an indicator. Methyl Red Acid Colour Base Colour Experiment On acetate ( an acid)… The indicator will be in its acidic form/colour. Indicator: Methyl Red… Turns from red to yellow 0 2 4 HIn HIn is Transition range 5 6 ⇄ H+ 7 + 8 10 InIn- is Transition Colour =5±1 = 4 to 6 12 [HInd] = [Ind-] 14 Indicators Chart Indicator Transition range Transition Colour Colour Change as pH Increases 4-6 orange Red to yellow intense Red 0 2 4 5 6 7 8 10 12 14 HIn ⇄ H+ + Red InYellow Le Chatelier’s Principle can be used to explain the colours of Red. In an acid solution, [H+] is high and the equilibrium shifts left and turns red. In a base solution, [H+] is low and it shifts right and turns yellow. At pH = 5…the transition point [HInd] = [Ind-] Orange(mixture of red and yellow) Look up Alizarin yellow. It tells you that it changes from yellow to red as pH increases. This means that the acid form is yellow and the base form is red. To calculate the transition point, you have to average the two pH values Transition Point = = 10.1 + 2 11.05 12.0 Alizarin Yellow HIn ⇄ Acid form yellow H+ + InBase form red Transition range 10.1 – 12.0 Transition point pH= 11.05 This means: The solution is yellow when pH < 11.05 The solution is red when pH > 11.05 Anything between 10.1 to 12.0 would be orange! Alizarin Yellow HIn ⇄ Acid form yellow H+ + InBase form red At pH = 11.05, the transition point, the: The solution looks orange, which is a blend of Ka = [H+][Ind-] [HInd] Ka = [H+] [HInd] = [Ind-] yellow and red. [HInd] = [Ind-] Only at the transition point Keep in mind… Transition point, or end point, is the point where the indicator is half way through its colour change. Equivalence point or stoichiometric point, is the point where mole ratio of the reaction is exactly equal to the mole ratio required by the stoichiometry of the reaction. Keep in mind… At the transition point of the indicator Ka for the indicator = [ H+] since [HIn] = [ In- ] From this, we can figure out the solution’s pH @ the transition point. Ex: Calculate the Ka for methyl orange. Transition point [H+] Ka = = pH 10-pH [H+] = = (3.2 + 4.4)/2 = 3.8 = 10-3.8 = 2.0 x 10-4 1.58 x 10-4 M Ex: An indicator has a Ka = 1.0 x 10-6, calculate the pH of the solution at the transition point. Ka = [H+] = 1.0 x 10-6 M pH = -log[H+] = = -Log[1.0 x 10-6] 6.00 The indicator is chlorophenol red because (5.2 + 6.8)/2 = 6.0 TRY: answer the following questions: A solution turns yellow when Orange IV is added and red when methyl orange is added. A) what is the pH range for the solution? B) what is pH transition ? C) what is [H30+] of solution? D) What is ka of indicator? TRY: Alizarin Yellow changes from yellow to red at pH = 11.0 what colour is Alizarin Yellow in 1 x 10-5 M NaOH? Universal Indicators • It is an indicator solution which changes colour several times over a range of pH values. – Colour changes are distinct enough to estimate pH of a solution within + 0.5 pH Summary of equations At the transition point of the indicator Ka for the indicator = [ H+] since [HIn] = [ In- ] pKa = pH From Ind. Table, average pH range to get pH transition. pH transition is a reference point for colours which means: pH solutions > pH transition –basic form of indicator predominates(base color) pH solutions < pH transition point – acidic form of indicator predominates(acid color) pH solution in between the two pH numbers on your chart, means the color is a mixture Practice Problems Page 162 • 108, 110, 112, 114, 116, 117 For #116, consider Thymol Blue on Bottom.