Pre-School Wheeze: Recent New Insights
advertisement

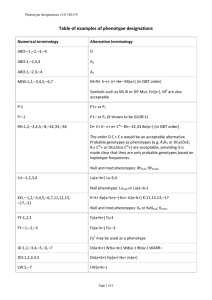
Grazie per aver scelto di utilizzare a scopo didattico questo materiale delle Guidelines 2011 libra. Le ricordiamo che questo materiale è di proprietà dell’autore e fornito come supporto didattico per uso personale. Phenotyping Severe Asthma in Children Andrew Bush MD FRCP FRCPCH Imperial College & Royal Brompton Hospital a.bush@imperial.ac.uk Phenotyping Children Why do it? • School age children: principles • School age children: phenotypes • Summary and Conclusions What is a phenotype? • A phenotype is here defined as a feature or cluster of features which differentiates a separate group from a defined population at a given time • Some useful action must result! – Understanding of mechanisms of disease – Results in a change of treatment – Helps with monitoring disease How Phenotype? • Investigator prejudice – Eosinophilic, neutrophilic, mixed, paucicellular • Self-fulfilling Self-fulfilling: Infant Wheezing Phenotypes • Never (51%) • Transient (20%) – Wheeze 0-3, not at age 6 • Persistent (14%) – Wheeze 0-3 still present age 6 • Late onset (15%) – Wheeze after age 3 How Phenotype? • Investigator prejudice – Eosinophilic, neutrophilic, mixed, paucicellular • Self-fulfilling • Mathematical techniques – PCA, latent class analysis – Systems biology Data driven: Infant Wheezing Phenotypes Atopy: a dichotomous variable? Patients and Methods • Birth cohort study age 5 years – Questionnaire n = 815 – SPT n = 717 – Specific IgE n = 478 Main Results • CR 26.1%, CRC 12-1% • Increased risk with greater sensitisation • Outcomes – Current rhinitis (CR) – Current rhinoconhunctivitis (CRC) Allergy 2007; 62: 1379-86 Conclusions • Atopy is not ‘all-or-none’ Grass IgE and current rhinitis Mite IgE and perennial rhinitis Grass IgE and rhinoconjunctivitis Grass IgE and seasonal rhinitis There is a dose effect for specific IgE and atopic manifestations 16S rRNA: The Sterile Airway? • 5054 16S rRNA from 43 subjects, > 70% bacterial species • Bronchial tree NOT sterile– 2000 sequences cm2 sampled • Proteobacteria more abundant in asthmatic children, prevotella in controls (same as adults) • There are more bugs in the lung than the gut! – – Think gastric acid! The gut is BETTER protected! PLoS One 2010; 51: e8578 Phenotyping Children • Why do it? School age children: principles • School age children: phenotypes • Summary and Conclusions Inflammometry: not for mild asthma Mild Severe Standard strategy Sputum strategy Standard strategy Sputum strategy ERJ 2006; 27: 483-94 New terminology and definitions Problematic Severe Asthma NB: is it asthma at all? NB: is it ‘asthma plus’ Stage 1 assessment Difficult asthma •Remediable factors identified •Therapy adherence addressed Genuine severe, therapy resistant asthma Lancet 2008; 372: 1019-21 Problematic Severe Asthma • Difficult asthma = – becomes easier when the basics are got right (adherence, environment, etc.) – NOT candidates for novel therapies • Severe, therapy-resistant asthma = – treatment still extremely difficult despite getting the basics right – Would be potentially suitable for cytokine specific therapies ‘Difficult’ vs. ‘Severe, Therapy resistant’ Asthma • Psycho-social issues re-addressed – Anecdotally, more likely to ‘open up’ – 74% referrals were after home discussions • Adherence • Smoking • Allergens Arch Dis Child 2009; 94: 780-4 Inflammatory pattern? Phenotype Discordance? Steroid Responsiveness? What is target lung function (PAL)? Next Step: FOB Assess symptoms, use of rescue medication Four weeks later: Decision time Assess symptoms, use of rescue medication Spirometry & reversibility Spirometry & reversibility Induced sputum, FeNO Induced sputum, FeNO FOB, BAL, biopsy Develop treatment plan Intramuscular triamcinolone Mucosal and Luminal Cytology No correlation between the two: which matters? Lex et al, BlueJ 2006; 174: 1286-91 Stability of Inflammatory Phenotypes over Time • Sputum obtained from 40 subjects with severe asthma, > twice over a one year period • Age range 8.4-17.6 years – 17 children (42%) showed a change in phenotype (between eosinophilic, neutrophilic and mixed) • What is a real change in phenotype? • How often should phenotypes be re-assessed? Fleming L, MD(Res) 2010 Phenotyping Children • Why do it? • School children: principles School age children: phenotypes • Summary and Conclusions RBH Paediatric Severe Asthma: Demographics (1) • N=71 (35 male), group mean age 11.9, range 4.5 - 17-5 • FP equivalent dose 1000 mcg (5003000); n=21, oral steroids • Admissions median 2, 0-21 (12 ventilated) Cladosporidium Alternaria Egg Milk Peanut Trees Aspergilus HDM Dog Cat Grasses 0 10 20 30 % positive %positive SPTs 40 50 60 RBH Paediatric Severe Asthma: Demographics (2) • FEV1 = 76% (33125) • BDR = 14% (12106) • ACT (25): – >20, 3% – 16-19, 25% – < 15, 72% Number of subjects • FeNO50 = 52ppb (5-171), NR <25ppb 15 10 5 0 30 40 50 60 70 80 90 100 Post bronchodilator FEV1 (% predicted) FEV1 % predicted 110 Serum Total IgE Ineligible for Anti IgE Percent of subjects (%) Frequency 40 30 G median 550 iu G mean 401 iu 20 normal 10 0 16 40 100 Serum IgE IgE log serum 251 630 1584 3980 Airway Phenotype (post-steroids) • BAL: – – – – 14/68 eosinophilic 19/68 neutrophilic 11/68 mixed cellularity 24/68 paucicellular • EBB – – – – 19/68 eosinophilic 19/68 neutrophilic 17/68 mixed cellularity 13/68 paucicellular ERJ 2009; 34: 1052-9 ENFUMOSA SPTs • 163 severe asthmatics, 158 mild (low dose ICS) – Median ICS 1773 – One third oral pred – Median pred 19 mg • Female preponderance (4.4:1 vs. 1.6:1 mild) • Severe asthma (hatched) – Less atopic – More PMNLs Eur Respir J 2003; 22: 470-7 Induced Sputum Adult and Paediatric Differences? 100 FEV 1/FVC 90 80 c mwb Nearly 50% SA now have COPD! Stronger signal than smoking 70 wb a sa 60 7 10 14 21 28 age at review (years) 35 42 Inflammatory pattern? Phenotype Discordance? Steroid Responsiveness? What is target lung function (PAL)? Next Step: FOB Assess symptoms, use of rescue medication Four weeks later: Decision time Assess symptoms, use of rescue medication Spirometry & reversibility Spirometry & reversibility Induced sputum, FeNO Induced sputum, FeNO FOB, BAL, biopsy Develop treatment plan Intramuscular triamcinolone Discordant Phenotypes • Individual variability in clinical, physiological & pathological parameters Am J Respir Crit Care Med 2008; 178: 218-24 Inflammometry and Treatment? • Pauci-inflammatory – Reduce or do not escalate anti-inflammatory therapy • Persistently eosinophilic – Omalizumab – Theophyllines – Steroid sparing agents (MTX, CyA) • Neutrophilic – Macrolides – Anti-LTB4 – Theophyllines • Mixed – ??? Persistent Airflow Limitation • Post-steroid trial, post acute BDR FEV1 < -1.96 Z-scores – What dose, route of administration and duration of steroids – What dose of bronchodilator? • No good paediatric evidence • BUT, no point flogging a dead horse! CAMP: Accelerated Airway Obstruction • MILD ASTHMATICS, BUT: – c25% had airway ‘failure to thrive’ – Independent of treatment (ICS, nedocromil, placebo) BlueJ 2004; 170: 234-41 Re-analysis of START trial • Does early intervention with ICS prevent exacerbations (initial question) – Lancet 2003; 361: 1071-6 • Once daily BUD 200mcg (children) or 400mcg (adults) vi Turbohaler™ vs. lactose placebo • 7241 patients aged 5-66 years from 32 countries • 1363/6767 received one course prednisolone – Placebo, n=818/3368 – BUD, n=545/3399 • What was the effect on ΔFEV1 (new, post hoc question) Results: Corticosteroid response Response Number (%) Symptom (ACT ≥ 20/25 or 50% increase) Lung Function (FEV1 ≥ 80% or 15% increase) 23/47 (49%) FeNO (FeNO50 < 24) 22/52 (42%) Sputum (Eo < 2.5%) 22/42 (52%) 29/52 (56%) Proposed criteria for corticosteroid response • Complete response: normalisation/ improvement in all parameters (symptoms, FEV1, FeNO, sputum); 8/52 (15%) • Partial: response in one or more of the parameters; 36/52 (69%) • Non: response in none of the parameters; 8/52 (15%) Other criteria for steroid response? • Clinical response; ACT and FEV1 normal or improved – 15/50 (30%) • Inflammatory response; Normal sputum cytology, or if no sputum available, FeNO normal – 26/52 (50%) Exacerbations vs. Baseline control • Exacerbation of Asthma – Linear decline, then linear recovery of PEFR – No increase in variability – Usually viral – Usually difficult to prevent • Loss of Asthma control – Wide diurnal variability BUD – Morning dips – BDR > 30% • These are not the same thing! Lancet 1999; 353: 364-9 Eosinophilic/Exacerbating • CAMP: 30% never exacerbated – N Engl J Med 2000;343:1054–1063 • Genetics different: CD14, CD16 – Am J Respir Crit Care Med. 2006; 173: 617-22 • Specific mucin glycan phenotype (O-secretor) – Am J Respir Crit Care Med 2011; 183; 189-94 • Eosinophilic, discordant phenotype Lessons from TENOR • Previous exacerbation STRONGLY predictive of future exacerbation – Independent of asthma control or duration – Use of controllers – Allergic triggers • Also predictive are: – Allergic triggers – NOT ‘All or none’ – Non-white race – Poorly controlled asthma (impairment domain) The exacerbating child: What actions to take? • We (as yet) cannot modulate viral infections! – Are they taking low dose ICS (care with escalation)? – Has baseline control been optimised? – Has baseline lung function been optimised? – What are their allergic triggers? – Has allergen exposure been reduced? – (Has eosinophilic airway inflammation been controlled?) Phenotyping Children • Why do it? • School age children : principles • School age children : phenotypes Summary and Conclusions Summary and Conclusions • Phenotyping has to be useful if it is to be justifiable – Understanding mechanisms – Guiding treatment • Get the basics right first • Childhood and adult disease differs • Be sure you are clear what is meant by ‘severe asthma’ in a given study • We need international collaborations We have a long way to go!