Measurements on Gases
advertisement

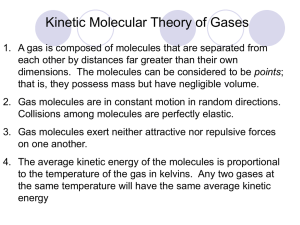
Chapter 5: Gases 5.1 Measurements on Gases • Volume- amount of space the gas occupies: 1 L = 1000 mL = 1000 cm3 = 1 x10-3 m3 • Amount – most commonly expressed in terms of moles (n): m = MM x n • Temperature – measured in degrees Celsius but commonly must convert to Kelvin: TK = t*C + 273.15 • Pressure – gas molecules are constantly colliding & because of this they exert a force over an area: 1.013 bar = 1 atm = 760 mmHg = 1 x 105 Pa = 14.7 psi Barometer Manometer Example 5.1 • A balloon with a volume of 2.06 L contains 0.368 g of helium at 22 degrees Celsius and 1.08 atm. Express the volume of the balloon in m3, the temperature in K, and the pressure in mmHg. – V = 2.06 x 10-3 m3 – nHe = 0.0919 mole – T = 22 + 273.15 = 295 K – P = 821 mmHg Gas Laws • Boyle’s Law: P1V1 = P2V2 • Charles’ Law: V1 = V2 T1 T2 • Gay-Lusaac’s Law: P1 = P2 T1 T2 • Combined Gas Law: P1V1 = P2V2 T1 T2 Example • A tank is filled with a gas to a pressure of 977 mmHg at 25*C. When the tank is heated, the pressure increases to 1.50 atm. To what temperature was the gas heated? – 75*C 5.2 The Ideal Gas Law & 5.3 Gas Law Calculations – The Ideal Gas Law Constant (R): 0.0821 L atm/mol K - ideal gas law problems 8.31 J/ mol K - equations involving energy 8.31 x 103 g m2/s2 mol k- molecular speed problems Molar Volume Initial & Final State Problems • Starting with a sample of gas at 25*C and 1.00 atm you might be asked to calculate the pressure developed when the sample is heated to 95*C at a constant volume. Determine a twopoint equation and solve for the final pressure. – Initial State: P1V = nRT1 – Final State: P2V = nRT2 – Divide the 2 equations to derive a “two-point” equation: • P1 = T1 P2 = T2 – Rearrange to solve for the variable you want: P2 = P1 T2 T1 Ans: 1.23 atm Example 5.2 • A 250.0 mL flask, open to the atmosphere, contains 0.0110 mol of air at 0 *C. On heating, part of the air escapes: how much remains in the flask at 100 *C? – 0.00805 mol of air Example 5.3 • If 2.50 g of sulfur hexafluoride is introduced into an evacuated 500.0 mL container at 83*C, what pressure (atm) is developed? – Ans: 1.00 atm Density & The Ideal Gas Law The ideal gas law offers a simple approach to the experimental determination of the molar mass of a gas. – Remember that m = MM x n and n = PV and d = m RT V – So you can substitute these equations into the ideal gas law to solve fro density (d) or molar mass (M) –Gas Density and Human Disasters: Many gases that are denser than air have been involved in natural and human-caused disasters. The dense gases in smog that blanket urban centers, such as Mexico City (see photo), contribute greatly to respiratory illness. In World War I, poisonous phosgene gas (COCl2) was used against ground troops as they lay in trenches. In 1984, the unintentional release of methylisocyanate from a Union Carbide India Ltd. chemical plant in Bhopal, India, killed thousands of people as vapors spread from the outskirts into the city. In 1986 in Cameroon, CO2 released naturally from Lake Nyos suffocated thousands as it flowed down valleys into villages. Some paleontologists suggest that a similar process in volcanic lakes may have contributed to dinosaur kills. Example 5.4 Acetone is widely used in nail polish remover. A sample of liquid acetone is placed in a 3.00 L flask and vaporized by heating to 95*C at 1.02 atm. The vapor filling the flask at this temperature and pressure weighs 5.87 g: (a) What is the density of acetone vapor under these conditions? Ans: 1.96 g/L (b) Calculate the molar mass of acetone. Ans: 58.1 g/mol (c) Acetone contains three elements C, H, and O. When 1.00 g of acetone is burned 2.27 g of CO2 and 0.932 g of H2O are formed. What is the molecular formula of acetone? Ans: C3H6O 5.3 Stoichiometry of Gaseous Reactions • A molar ratio from a balanced chemical reaction is also used in reactions involving gases however, the ideal gas law can now be applied. • Example 5.5: A nickel smelter in Sudbury, Ontario produces 1% of the world’s supply of sulfur dioxide by the reaction of nickel II sulfide with oxygen another product of the reaction is nickel II oxide: What volume of sulfur dioxide at 25*C and a pressure of one bar is produced from a metric ton of nickel II sulfide? Ans: 2.73 x 105 L Gas A to Gas B Example 5.6 • Octane, C8H18, is one of the hydrocarbons in gasoline. On combustion octane produces carbon dioxide and water. How many liters of oxygen, measured at 0.974 atm and 24*C, are required to burn 1.00 g of octane? – Ans: 2.73 L Law of Combining Volumes • The volume of any 2 gases in a reaction at constant temperature and pressure is the same as the reacting molar ratio: 2 H2O (l) 2H2 (g) + O2 (g) 4 L H2 x 1 L O2 = 2 L O2 2 L H2 Example 5.7 • Consider the reaction for the formation of water from its elemental units. – (a) What volume of hydrogen gas at room temperature and 1.00 atm is required to react with 1.00 L of oxygen at the same temperature and pressure? • Ans: 2.00 L hydrogen gas – (b) What volume of water at 25*C and 1.00 atm (d=0.997 /mL) is formed from the reaction in (a)? • Ans: 1.48 mL of water – (c) What mass of water is formed from the reaction assuming a yield of 85.2%? • Ans: 1.26 g of water Limiting Reactant Problems The alkali metals react with the halogens to form ionic metal halides. What mass of potassium chloride forms when 5.25 L of chlorine gas at 0.950 atm and 293 K reacts with 17.0 g of potassium? Ans: 30.9 g KCl 5.5 Dalton’s Law of Partial Pressures • The total pressure of a gas mixture is the sum of the partial pressures of the components of the mixture. Ptot = PA + PB +….. PH2 = 2.46 atm PHe = 3.69 atm then Ptot = 6.15 atm Wet Gases • When a gas is collected by bubbling through water then it picks up water vapor. Then the total pressure is the sum of the pressure of the water vapor and the gas collected. So Dalton’s Law can be applied by: Ptot = PH2O + PA *The partial pressure of water is equal to the vapor pressure of water. This has a fixed value at a given temperature (PH2O @ 25*C = 23.76 mmHg) Gas collection by water displacement. Example 5.8 • A student prepares a sample of hydrogen gas by electrolyzing water at 25*C. She collects 152 mL of hydrogen gas at a total pressure of 758 mmHg. Calculate: – (a) the partial pressure of hydrogen gas. • Ans: 734 mmHg – (b) the number of moles of hydrogen gas collected. • Ans: 0.00600 mol of hydrogen gas Partial Pressures & Mole Fraction • The partial pressure of a gas (PA) divided by the total pressure (Ptot) is equal to the number of moles of that gas divided by the total moles of gases: • PA = nA Ptot ntot • Mole fraction: XA = nA ntot • Partial Pressures: PA = XA Ptot Example 5.9 • Methane burns in air. When one mole of methane and four moles of oxygen are heated: (a) What are the mole fractions of oxygen, carbon dioxide, and water vapor in the resulting mixture (assume all the methane is converted)? XCH4 = 0, XCO2 = 0.200, XH2O =0.400, XO2 = 0.400 (b) If the total pressure of the mixture is 1.26 atm, what are the partial pressures of each gas? PCO2 = 0.252 atm, PH2O =0.504 atm, PO2 = 0.504 atm 5.6 Kinetic Theory of Gases The Molecular Model of Gases (pg 115): • Gases are mostly empty space (assumes that gases do not have their own volume). • Gas molecules are in constant and chaotic motion. Their velocities are constantly changing because of this. • Collisions of gases are elastic (assumes no attractive forces). • Gas pressure is caused by collisions of molecules with the walls of the container. As a result, pressure increases with the energy and frequency of these collisions. Average Speed • The equation below is derived from the average translational kinetic energy of a gas molecule: • It follows that at a given temperature, molecules of different gases have the same average kinetic energy of translational motion and • the average translational kinetic energy of a gas molecule is directly proportional to the Kelvin temperature so that: u = (3RT) ½ (MM) * An R value of 8.31 x 103 g m2/(s2 mol K) is used for average speed calculations. Graham’s Law of Effusion • The average speed is inversely proportional to the square root of the molar mass (MM). So for two different gases A and B at the same temperature then we can write: rate of effusion B = (MMA) 1/2 rate of effusion A = (MMB) Example: Using Graham’s Law A mixture of helium (He) and methane (CH4) is placed in an effusion apparatus. Calculate the ratio of their effusion rates: Ans: He effuses 2.002 times faster than methane Example 5.11 In an effusion experiment, argon gas is allowed to expand through a tiny opening into an evacuated flask of volume 120.0 mL for 32.0 s, at which point the pressure in the flask is found to be 12.5 mmHg. This experiment is repeated with a gas X of unknown molar mass at the same T and P. It is found that the pressure in the flask builds up to 12.5 mmHg after 48.0 s. Calculate the molar mass of X. Ans: 89.9 g/mol Real Gases • The ideal gas law has been used with the assumption that it applies exactly. However, all real gases deviate at least slightly from the ideal gas law. • These deviations arise because the ideal gas law neglects two factors: – 1. attractive forces between gas particles – 2. the finite volume of gas particles *In general, the closer a gas is to the liquid state, the more it will deviate from the ideal gas law. Correction for Real Gas Behavior